Chapter 20 Linear mixed effects models 4
20.1 Learning goals
- Some worked examples.
- Doing follow-up tests with the
emmeanspackage - Simulating, plotting, and analyzing models with different random effects structures
- Bootstrapping confidence intervals for fixed effects
- Logistic mixed effects model
20.2 Load packages and set plotting theme
library("knitr") # for knitting RMarkdown
library("kableExtra") # for making nice tables
library("janitor") # for cleaning column names
library("broom.mixed") # for tidying up linear mixed effects models
library("lme4") # for linear mixed effects models
library("afex") # for ANOVAs
library("car") # for ANOVAs
library("datarium") # for ANOVA dataset
library("modelr") # for bootstrapping
library("boot") # also for bootstrapping
library("ggeffects") # for plotting marginal effects
library("emmeans") # for marginal effects
library("tidyverse") # for wrangling, plotting, etc. 20.3 Load data sets
20.3.1 Sleep data
# load sleepstudy data set
df.sleep = sleepstudy %>%
as_tibble() %>%
clean_names() %>%
mutate(subject = as.character(subject)) %>%
select(subject, days, reaction)
# add two fake participants (with missing data)
df.sleep = df.sleep %>%
bind_rows(tibble(subject = "374",
days = 0:1,
reaction = c(286, 288)),
tibble(subject = "373",
days = 0,
reaction = 245))20.3.4 Politness data
Rows: 84 Columns: 5
── Column specification ─────────────────────────────────────────────────────────────────────────────────────────────────────────────
Delimiter: ","
chr (3): subject, gender, attitude
dbl (2): scenario, frequency
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.20.4 Follow-up tests with emmeans
Just like with the linear model lm(), we can use linear contrasts to test more specific hypotheses with lmer(). The emmeans() function from the emmeans package will be our friend.
20.4.1 Sleep study
Let’s ask some more specific question aboust the sleep study.
- Do reaction times differ between day 0 and the first day of sleep deprivation?
- Do reaction times differ between the first and the second half of the study?
Let’s visualize the data first:
ggplot(data = df.sleep %>%
mutate(days = as.factor(days)),
mapping = aes(x = days,
y = reaction)) +
geom_point(position = position_jitter(width = 0.1),
alpha = 0.1) +
stat_summary(fun.data = "mean_cl_boot")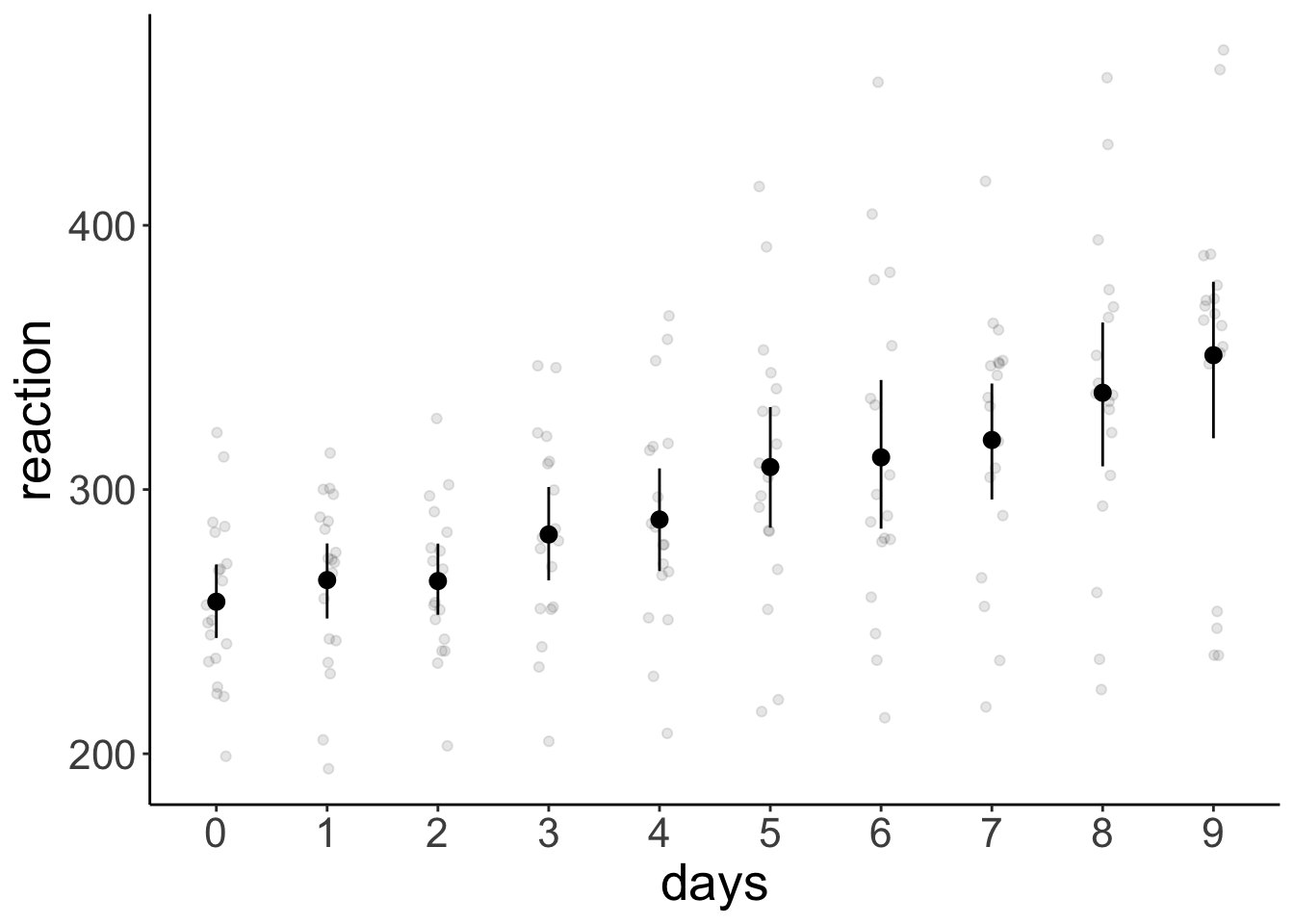
And now let’s fit the model, and compute the contrasts:
fit = lmer(formula = reaction ~ 1 + days + (1 | subject),
data = df.sleep %>%
mutate(days = as.factor(days)))
contrast = list(first_vs_second = c(-1, 1, rep(0, 8)),
early_vs_late = c(rep(-1, 5)/5, rep(1, 5)/5))
fit %>%
emmeans(specs = "days",
contr = contrast) %>%
pluck("contrasts") contrast estimate SE df t.ratio p.value
first_vs_second 7.82 10.10 156 0.775 0.4398
early_vs_late 53.66 4.65 155 11.534 <.0001
Degrees-of-freedom method: kenward-roger df.sleep %>%
# filter(days %in% c(0, 1)) %>%
group_by(days) %>%
summarize(reaction = mean(reaction))# A tibble: 10 × 2
days reaction
<dbl> <dbl>
1 0 258.
2 1 266.
3 2 265.
4 3 283.
5 4 289.
6 5 309.
7 6 312.
8 7 319.
9 8 337.
10 9 351.df.sleep %>%
mutate(index = ifelse(days %in% 0:4, "early", "late")) %>%
group_by(index) %>%
summarize(reaction = mean(reaction))# A tibble: 2 × 2
index reaction
<chr> <dbl>
1 early 272.
2 late 325.20.4.2 Weight loss study
For the weight loss data set, we want to check:
- Whether there was a difference between the first two vs. the last time point.
- Whether there was a linear trend across the time points.
Let’s first visualize again:
ggplot(data = df.weightloss,
mapping = aes(x = timepoint,
y = score,
group = diet,
color = diet)) +
geom_point(position = position_jitterdodge(dodge.width = 0.5,
jitter.width = 0.1,
jitter.height = 0),
alpha = 0.1) +
stat_summary(fun.data = "mean_cl_boot",
position = position_dodge(width = 0.5)) +
facet_wrap(~ exercises) +
scale_color_brewer(palette = "Set1")
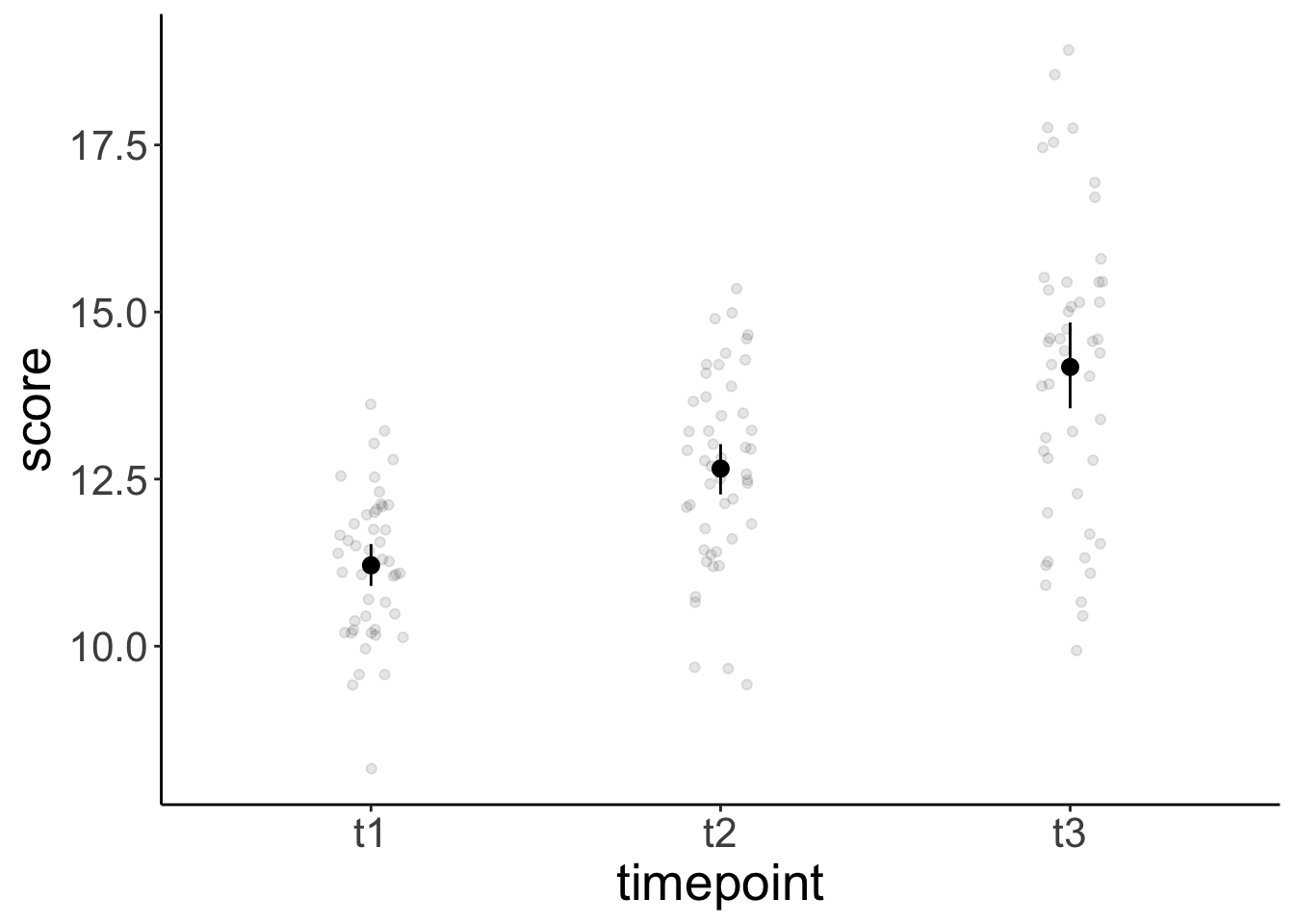
ggplot(data = df.weightloss,
mapping = aes(x = timepoint,
y = score)) +
geom_point(position = position_jitter(width = 0.1),
alpha = 0.1) +
stat_summary(fun.data = "mean_cl_boot") +
scale_color_brewer(palette = "Set1")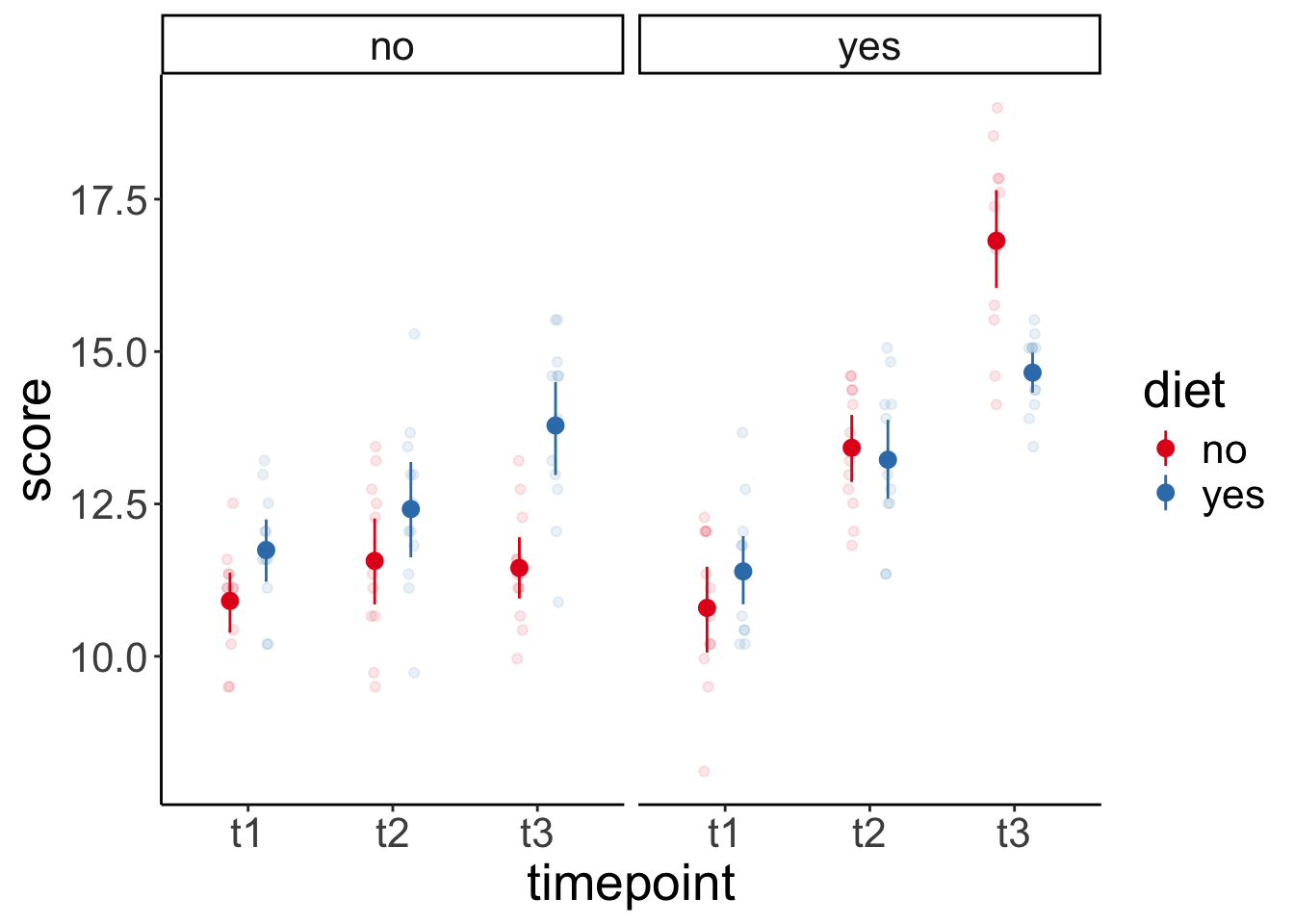
 And then fit the model, and compute the contrasts:
And then fit the model, and compute the contrasts:
fit = aov_ez(id = "id",
dv = "score",
between = "exercises",
within = c("diet", "timepoint"),
data = df.weightloss)Contrasts set to contr.sum for the following variables: exercisescontrasts = list(first_two_vs_last = c(-0.5, -0.5, 1),
linear_increase = c(-1, 0, 1))
fit %>%
emmeans(spec = "timepoint",
contr = contrasts)$emmeans
timepoint emmean SE df lower.CL upper.CL
t1 11.2 0.169 22 10.9 11.6
t2 12.7 0.174 22 12.3 13.0
t3 14.2 0.182 22 13.8 14.6
Results are averaged over the levels of: exercises, diet
Confidence level used: 0.95
$contrasts
contrast estimate SE df t.ratio p.value
first_two_vs_last 2.24 0.204 22 11.016 <.0001
linear_increase 2.97 0.191 22 15.524 <.0001
Results are averaged over the levels of: exercises, diet Because we only had one observation in each cell of our design, the ANOVA was appropriate here (no data points needed to be aggregated).
Both contrasts are significant.
20.4.3 Politeness study
For the politeness study, we’ll be interested in one particular contrast:
- Was there an effect of attitude on frequency for female participants?
Let’s visualize first:
# overview of the data
ggplot(data = df.politeness,
mapping = aes(x = attitude,
y = frequency,
group = gender,
color = gender)) +
geom_point(position = position_jitter(width = 0.1),
alpha = 0.1) +
stat_summary(fun.data = "mean_cl_boot") +
scale_color_brewer(palette = "Set1")Warning: Removed 1 row containing non-finite outside the scale range
(`stat_summary()`).Warning: Removed 1 row containing missing values or values outside the scale
range (`geom_point()`).# variation across scenarios
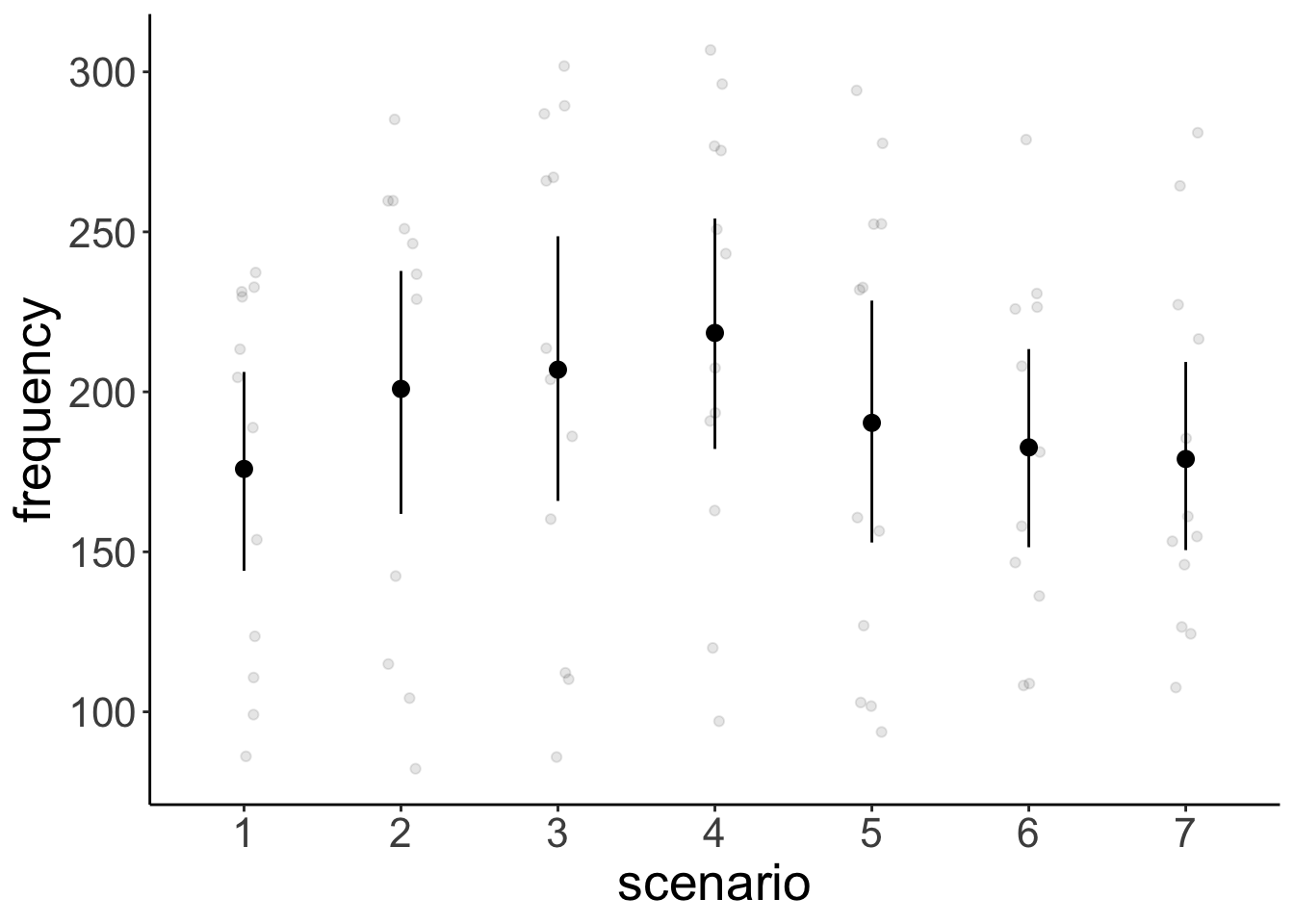
ggplot(data = df.politeness,
mapping = aes(x = scenario,
y = frequency)) +
geom_point(position = position_jitter(width = 0.1),
alpha = 0.1) +
stat_summary(fun.data = "mean_cl_boot") +
scale_color_brewer(palette = "Set1")Warning: Removed 1 row containing non-finite outside the scale range (`stat_summary()`).
Removed 1 row containing missing values or values outside the scale range (`geom_point()`).# variation across participants
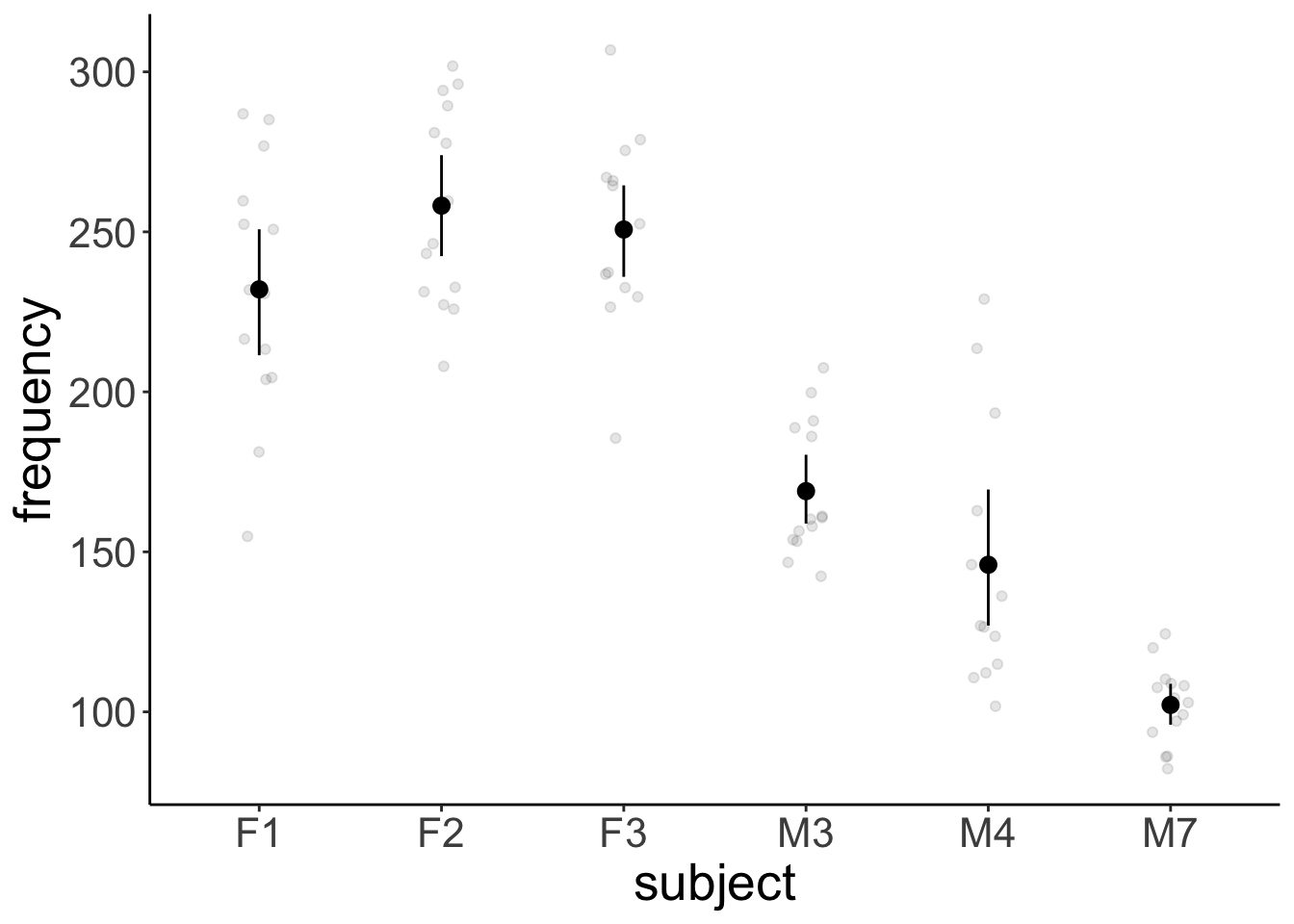
ggplot(data = df.politeness,
mapping = aes(x = subject,
y = frequency)) +
geom_point(position = position_jitter(width = 0.1),
alpha = 0.1) +
stat_summary(fun.data = "mean_cl_boot") +
scale_color_brewer(palette = "Set1")Warning: Removed 1 row containing non-finite outside the scale range (`stat_summary()`).
Removed 1 row containing missing values or values outside the scale range (`geom_point()`).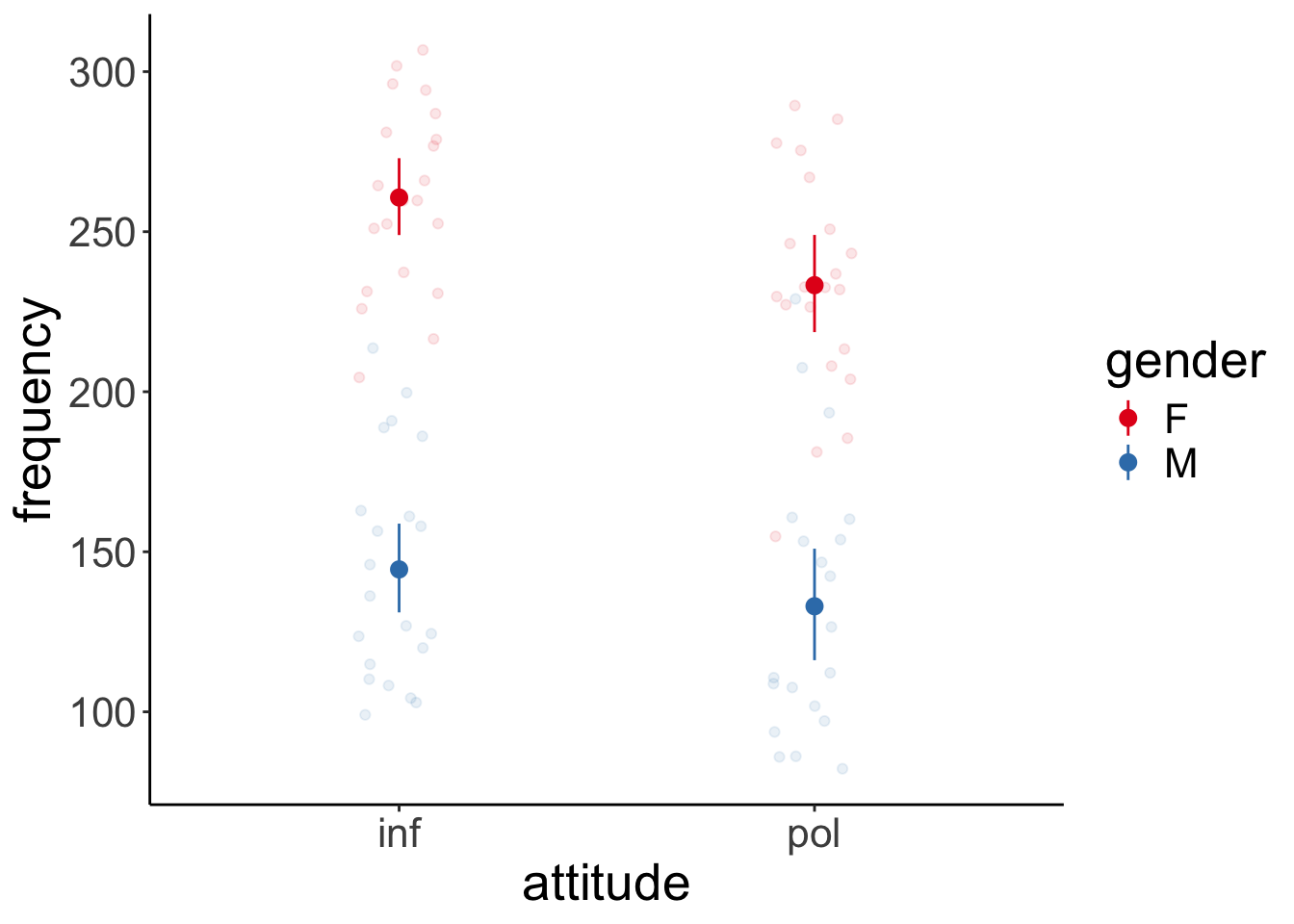
We fit the model and compute the contrasts.
fit = lmer(formula = frequency ~ 1 + attitude * gender +
(1 + attitude | subject) +
(1 + attitude | scenario),
data = df.politeness)
fit %>%
joint_tests() model term df1 df2 F.ratio p.value
attitude 1 3.20 9.522 0.0495
gender 1 4.00 26.600 0.0067
attitude:gender 1 3.99 1.997 0.2305$emmeans
attitude gender emmean SE df lower.CL upper.CL
inf F 261 16.0 4.97 219.4 302
pol F 233 16.8 5.17 190.5 276
inf M 144 16.0 4.97 103.2 186
pol M 133 16.9 5.23 89.8 175
Degrees-of-freedom method: kenward-roger
Confidence level used: 0.95
$contrasts
contrast estimate SE df t.ratio p.value
inf F - pol F 27.4 8.35 4.08 3.283 0.0295
inf F - inf M 116.2 21.30 4.00 5.448 0.0055
inf F - pol M 128.1 22.00 4.73 5.824 0.0025
pol F - inf M 88.8 21.90 4.70 4.046 0.0112
pol F - pol M 100.7 22.10 4.00 4.551 0.0104
inf M - pol M 11.9 8.46 4.28 1.405 0.2283
Degrees-of-freedom method: kenward-roger Here, I’ve computed all pairwise contrasts. We were only interested in one: inf F - pol F and that one is significant. So the frequency of female participants’ pitch differed between the informal and polite condition.
If we had used an ANOVA approach for this data set, we could have done it like so:
aov_ez(id = "subject",
dv = "frequency",
between = "gender",
within = "attitude",
data = df.politeness)Converting to factor: genderWarning: More than one observation per design cell, aggregating data using `fun_aggregate = mean`.
To turn off this warning, pass `fun_aggregate = mean` explicitly.Warning: Missing values for 1 ID(s), which were removed before analysis:
M4
Below the first few rows (in wide format) of the removed cases with missing data.
subject gender pol inf
# 5 M4 M NA 146.3Contrasts set to contr.sum for the following variables: genderAnova Table (Type 3 tests)
Response: frequency
Effect df MSE F ges p.value
1 gender 1, 3 1729.42 17.22 * .851 .025
2 attitude 1, 3 3.65 309.71 *** .179 <.001
3 gender:attitude 1, 3 3.65 21.30 * .015 .019
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '+' 0.1 ' ' 1This approach ignores the variation across scenarios (and just computed the mean instead). Arguably, the lmer() approach is better here as it takes all of the data into account.
20.5 Mixtures of participants
What if we have groups of participants who differ from each other? Let’s generate data for which this is the case.
# make example reproducible
set.seed(1)
sample_size = 20
b0 = 1
b1 = 2
sd_residual = 0.5
sd_participant = 0.5
mean_group1 = 1
mean_group2 = 10
df.mixed = tibble(
condition = rep(0:1, each = sample_size),
participant = rep(1:sample_size, 2)) %>%
group_by(participant) %>%
mutate(group = sample(1:2, size = 1),
intercept = ifelse(group == 1,
rnorm(n(), mean = mean_group1, sd = sd_participant),
rnorm(n(), mean = mean_group2, sd = sd_participant))) %>%
group_by(condition) %>%
mutate(value = b0 + b1 * condition + intercept + rnorm(n(), sd = sd_residual)) %>%
ungroup %>%
mutate(condition = as.factor(condition),
participant = as.factor(participant))20.5.0.1 Ignoring mixture
Let’ first fit a model that ignores the fact that there are two different groups of participants.
# fit model
fit.mixed = lmer(formula = value ~ 1 + condition + (1 | participant),
data = df.mixed)
summary(fit.mixed)Linear mixed model fit by REML. t-tests use Satterthwaite's method [
lmerModLmerTest]
Formula: value ~ 1 + condition + (1 | participant)
Data: df.mixed
REML criterion at convergence: 163.5
Scaled residuals:
Min 1Q Median 3Q Max
-1.62997 -0.41663 -0.05607 0.54750 1.54023
Random effects:
Groups Name Variance Std.Dev.
participant (Intercept) 19.2206 4.3841
Residual 0.3521 0.5934
Number of obs: 40, groups: participant, 20
Fixed effects:
Estimate Std. Error df t value Pr(>|t|)
(Intercept) 5.8729 0.9893 19.3449 5.937 9.54e-06 ***
condition1 1.6652 0.1876 19.0000 8.875 3.47e-08 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr)
condition1 -0.095Let’s look at the model’s predictions:
fit.mixed %>%
augment() %>%
clean_names() %>%
ggplot(data = .,
mapping = aes(x = condition,
y = value,
group = participant)) +
geom_point(alpha = 0.5) +
geom_line(alpha = 0.5) +
geom_point(aes(y = fitted),
color = "red") +
geom_line(aes(y = fitted),
color = "red")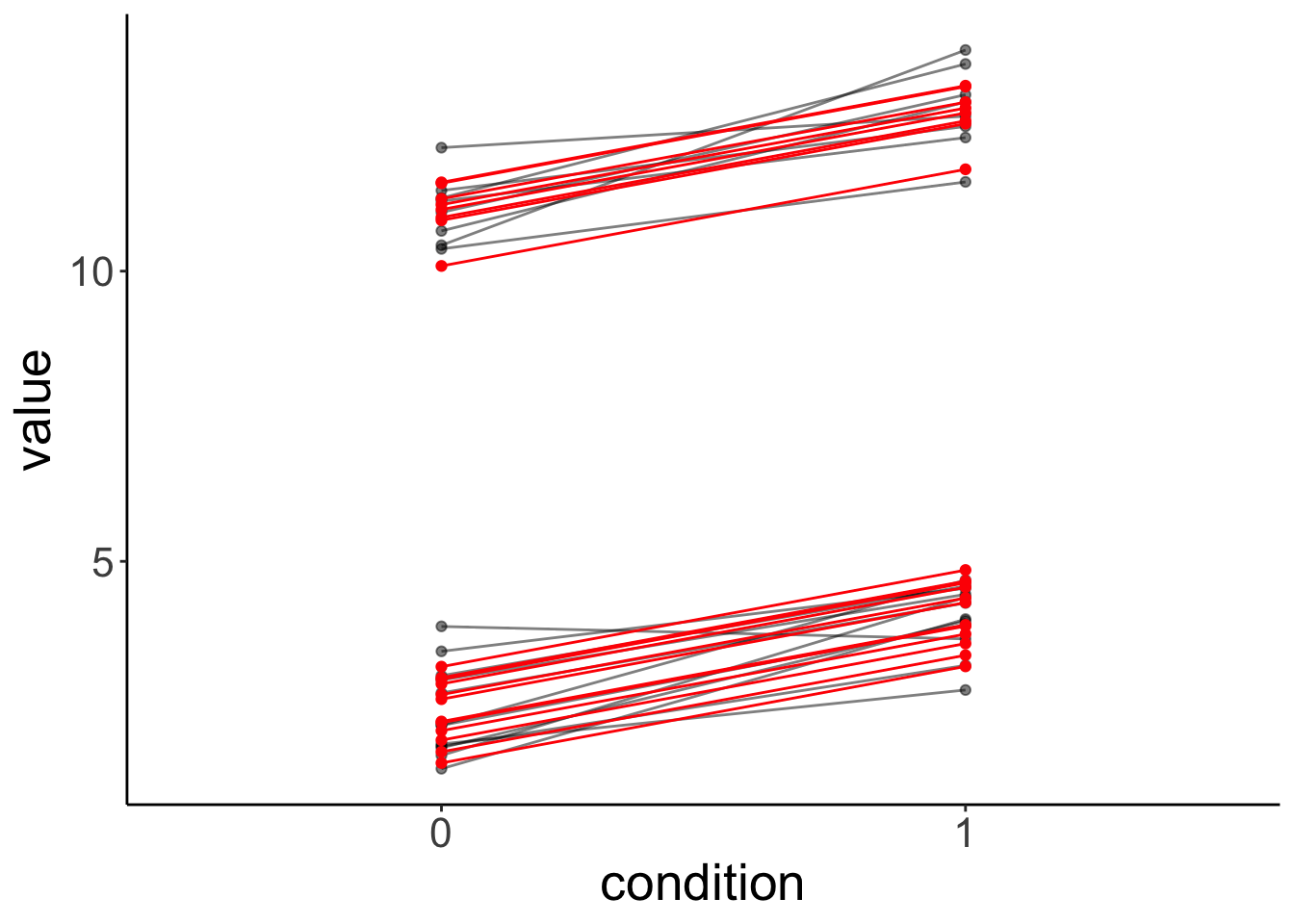
And let’s simulate some data from the fitted model:
# simulated data
fit.mixed %>%
simulate() %>%
bind_cols(df.mixed) %>%
ggplot(data = .,
mapping = aes(x = condition,
y = sim_1,
group = participant)) +
geom_line(alpha = 0.5) +
geom_point(alpha = 0.5)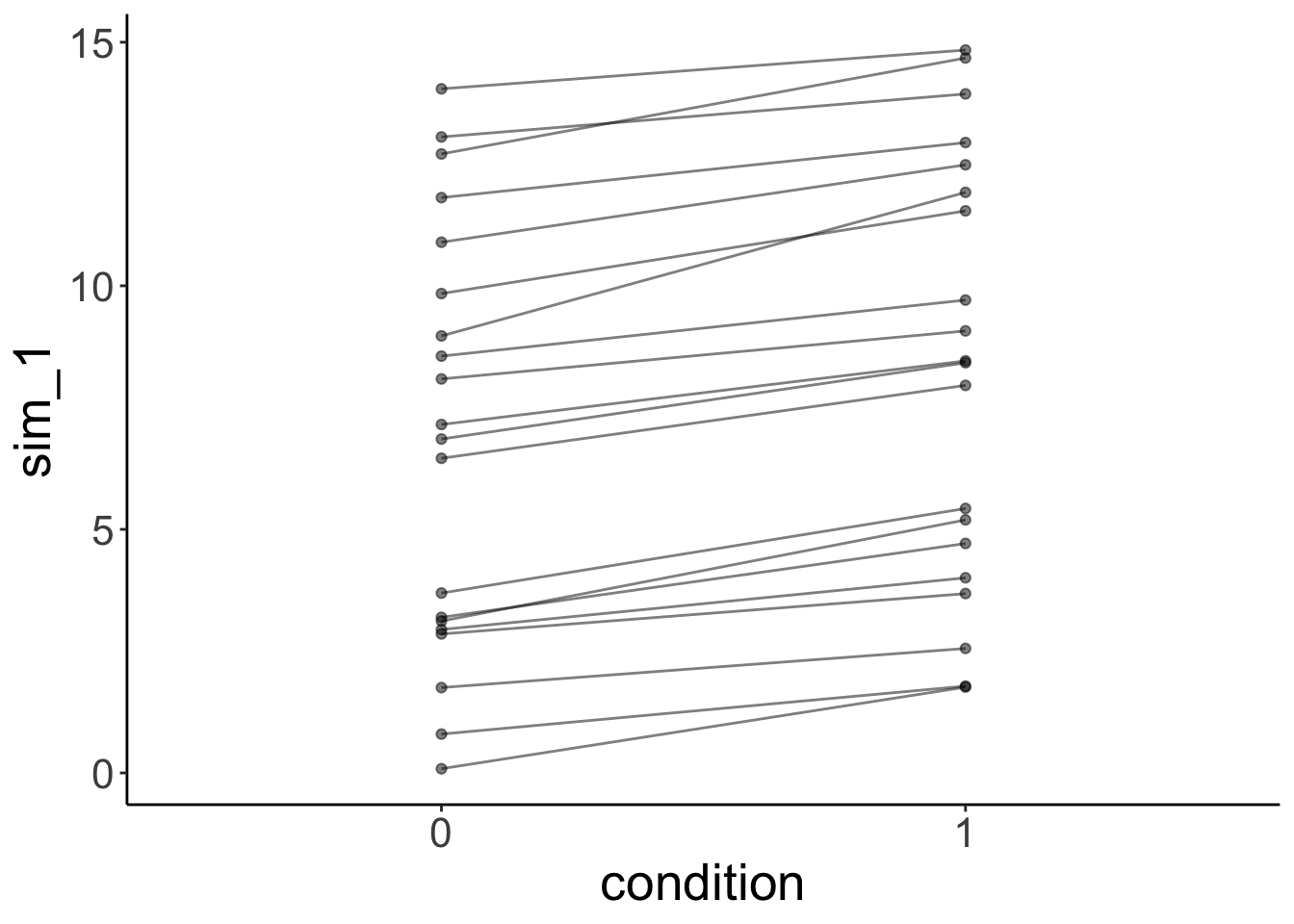
As we can see, the simulated data doesn’t look like the data that was used to fit the model.
20.5.0.2 Modeling mixture
Now, let’s fit a model that takes the differences between groups into account by adding a fixed effect for group.
# fit model
fit.grouped = lmer(formula = value ~ 1 + group + condition + (1 | participant),
data = df.mixed)
summary(fit.grouped)Linear mixed model fit by REML. t-tests use Satterthwaite's method [
lmerModLmerTest]
Formula: value ~ 1 + group + condition + (1 | participant)
Data: df.mixed
REML criterion at convergence: 82.2
Scaled residuals:
Min 1Q Median 3Q Max
-1.61879 -0.61378 0.02557 0.49842 2.19076
Random effects:
Groups Name Variance Std.Dev.
participant (Intercept) 0.09265 0.3044
Residual 0.35208 0.5934
Number of obs: 40, groups: participant, 20
Fixed effects:
Estimate Std. Error df t value Pr(>|t|)
(Intercept) -6.3136 0.3633 20.5655 -17.381 9.10e-14 ***
group 8.7046 0.2366 18.0000 36.791 < 2e-16 ***
condition1 1.6652 0.1876 19.0000 8.875 3.47e-08 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr) group
group -0.912
condition1 -0.258 0.000Note how the variance of the random intercepts is much smaller now that we’ve taken the group structure in the data into account.
Let’s visualize the model’s predictions:
fit.grouped %>%
augment() %>%
clean_names() %>%
ggplot(data = .,
mapping = aes(x = condition,
y = value,
group = participant)) +
geom_point(alpha = 0.5) +
geom_line(alpha = 0.5) +
geom_point(aes(y = fitted),
color = "red") +
geom_line(aes(y = fitted),
color = "red")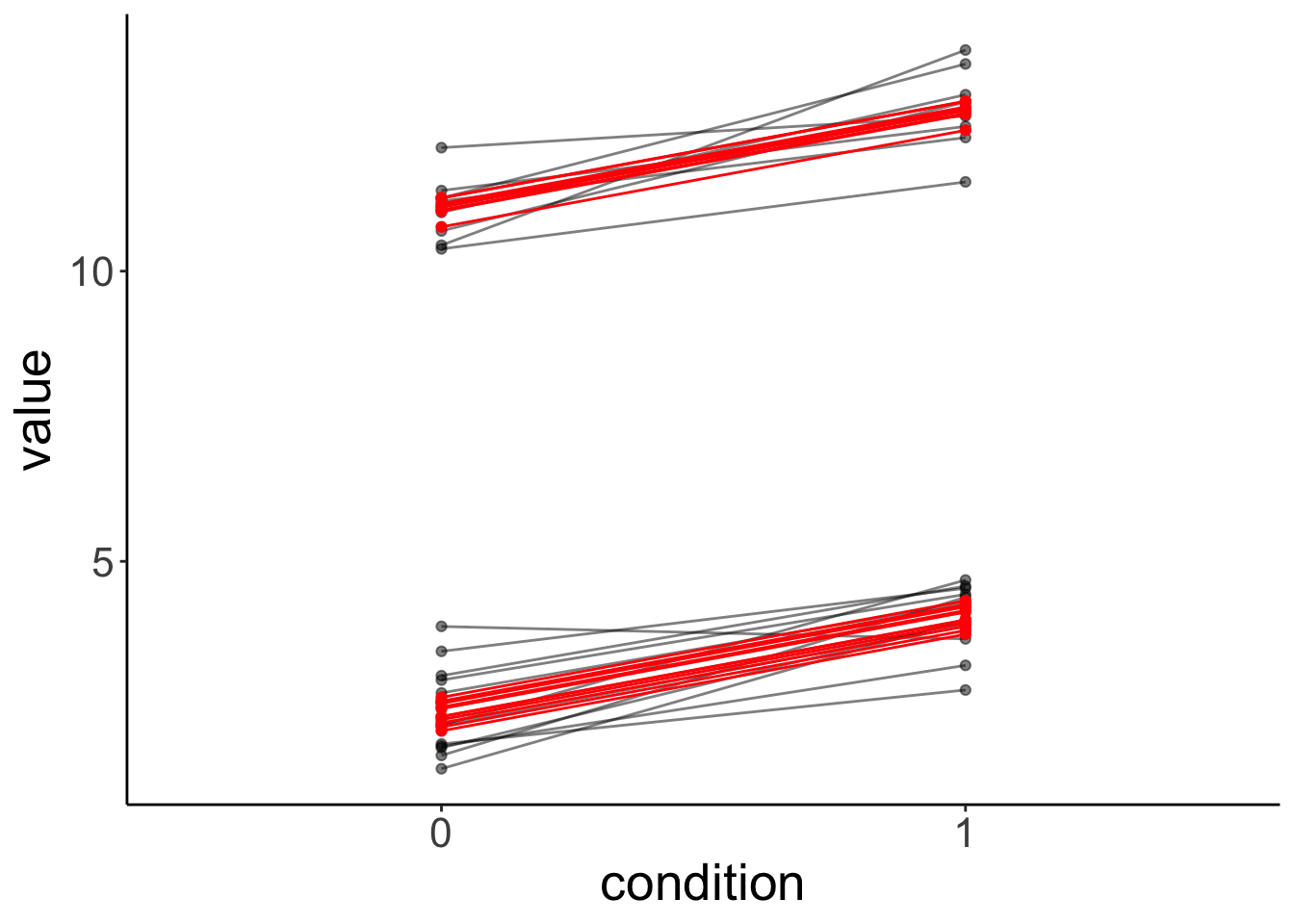
And simulate some data from the model:
# simulated data
fit.grouped %>%
simulate() %>%
bind_cols(df.mixed) %>%
ggplot(data = .,
mapping = aes(x = condition,
y = sim_1,
group = participant)) +
geom_line(alpha = 0.5) +
geom_point(alpha = 0.5)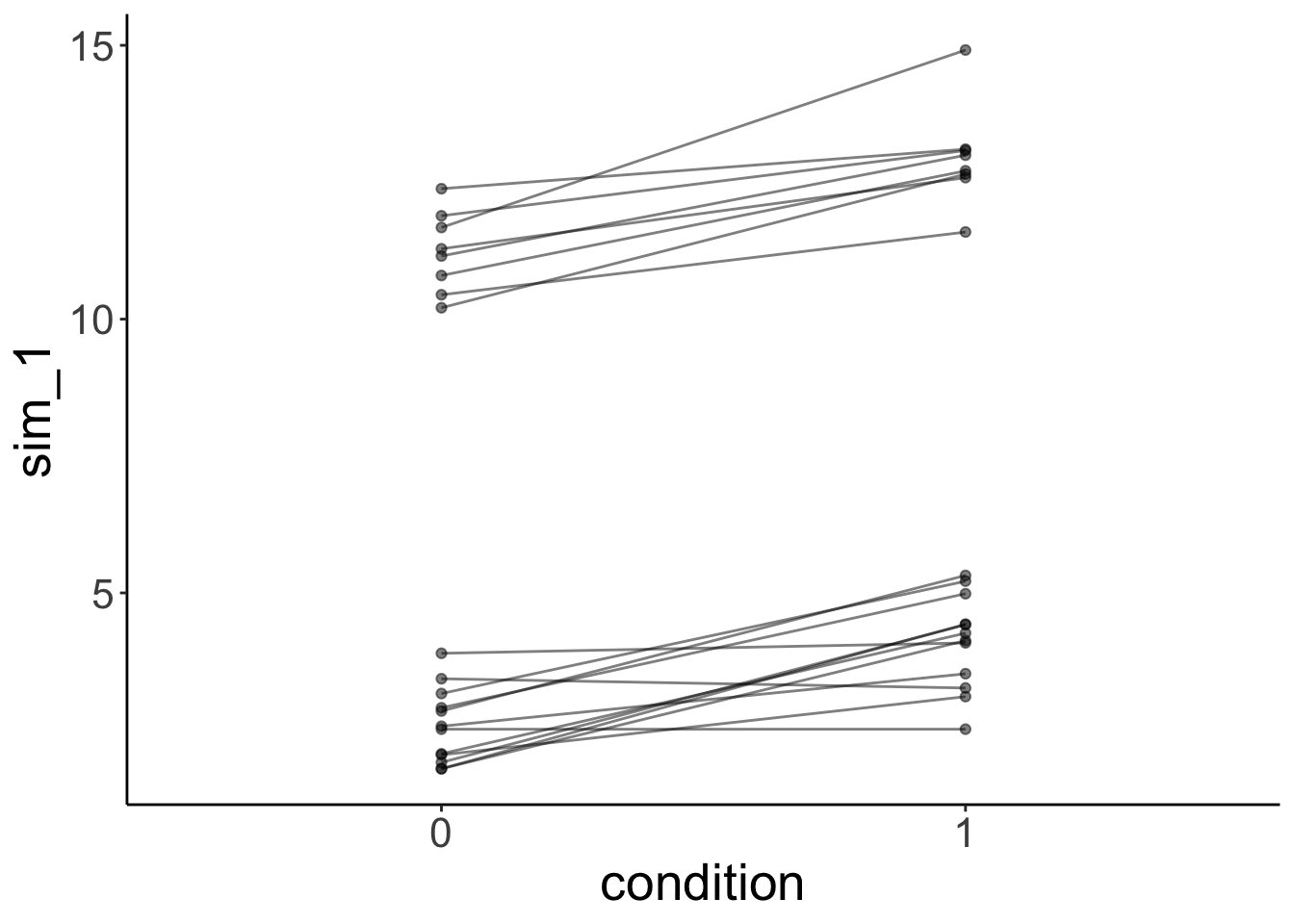
This time, the simulated data looks much more like the data that was used to fit the model. Yay!
ggpredict(model = fit.grouped,
terms = "condition") %>%
plot()
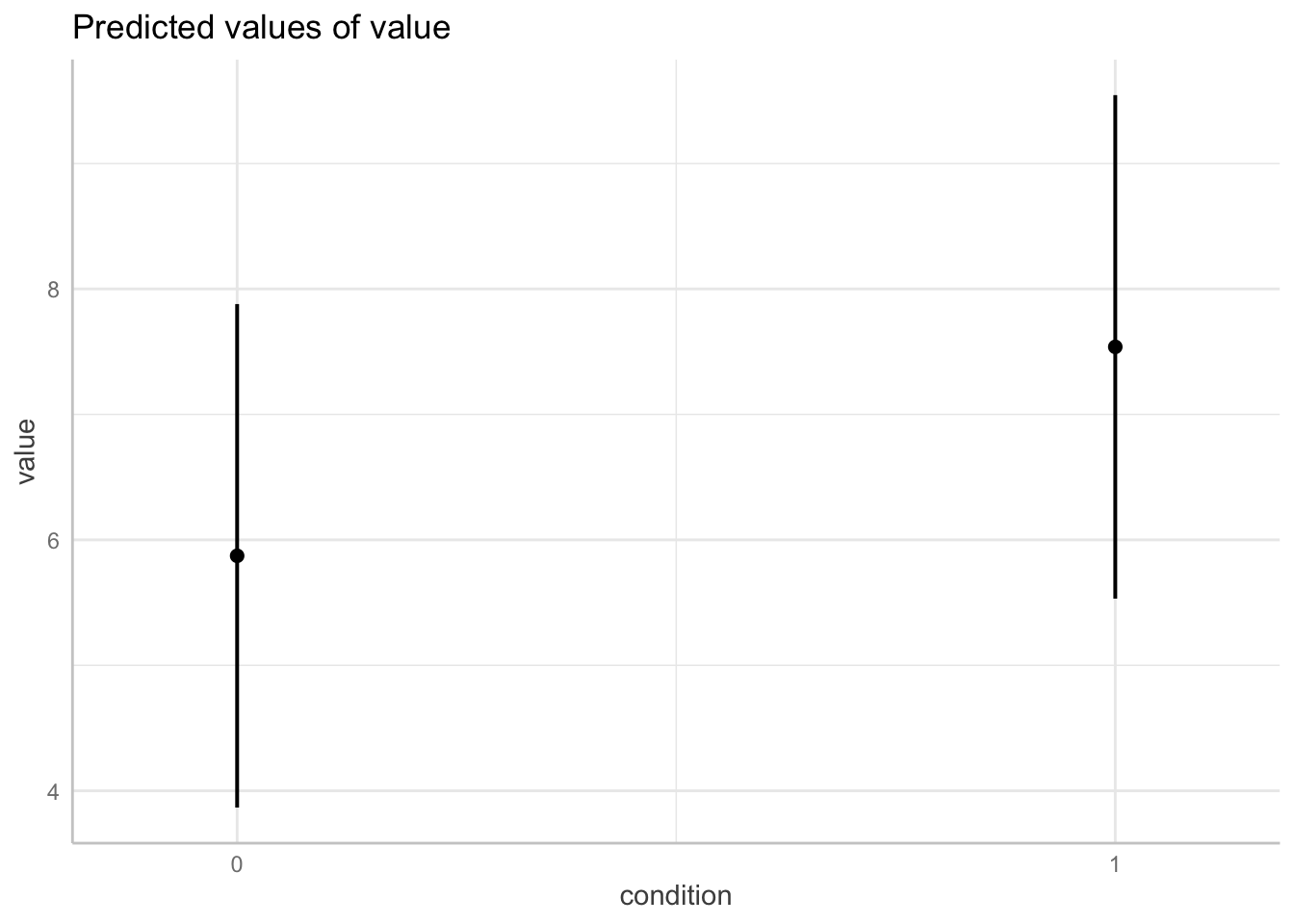
ggpredict(model = fit.mixed,
terms = "condition") %>%
plot()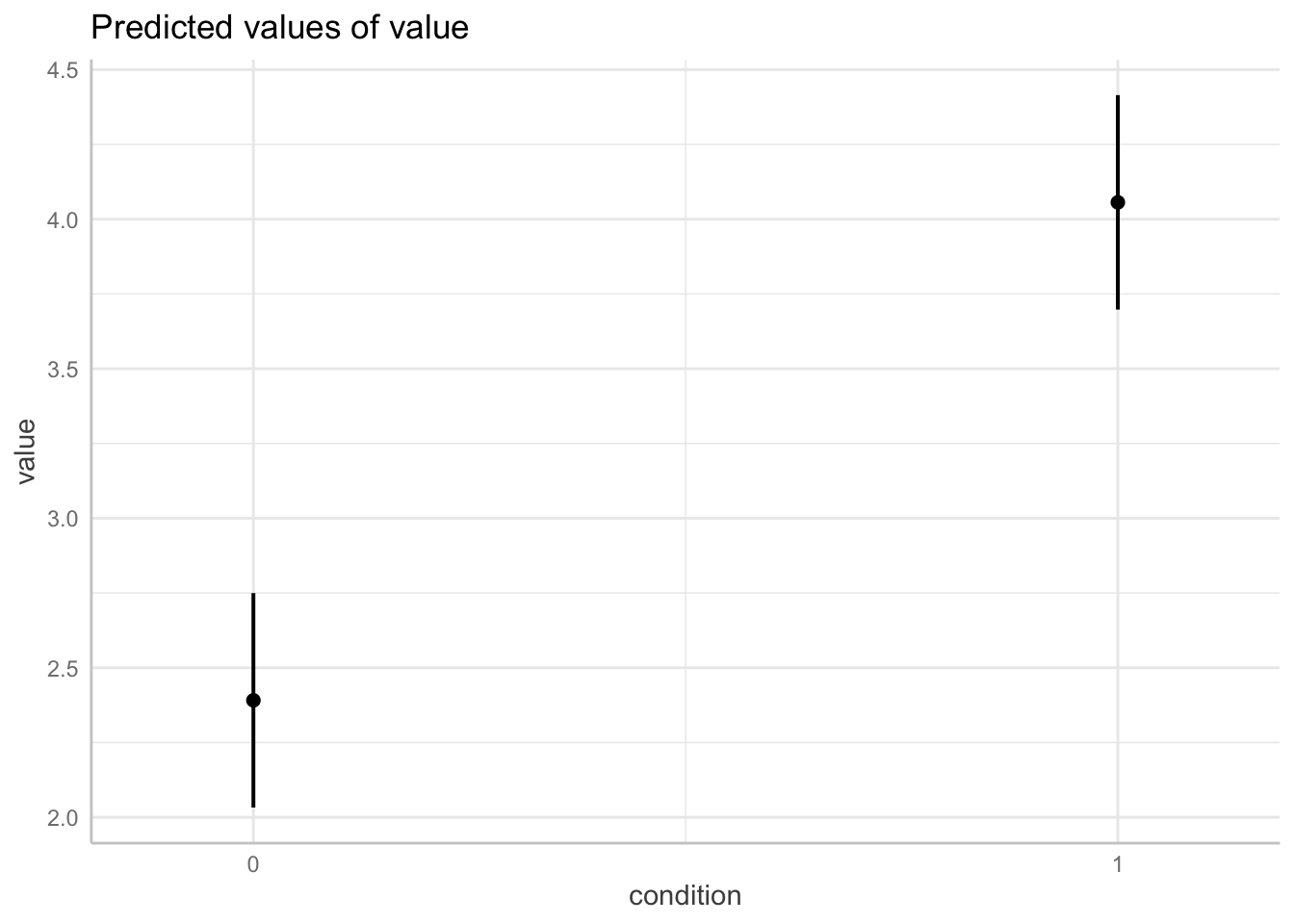
20.5.0.3 Heterogeneity in variance
The example above has shown that we can take overall differences between groups into account by adding a fixed effect. Can we also deal with heterogeneity in variance between groups? For example, what if the responses of one group exhibit much more variance than the responses of another group?
Let’s first generate some data with heterogeneous variance:
# make example reproducible
set.seed(1)
sample_size = 20
b0 = 1
b1 = 2
sd_residual = 0.5
mean_group1 = 1
sd_group1 = 1
mean_group2 = 30
sd_group2 = 10
df.variance = tibble(
condition = rep(0:1, each = sample_size),
participant = rep(1:sample_size, 2)) %>%
group_by(participant) %>%
mutate(group = sample(1:2, size = 1),
intercept = ifelse(group == 1,
rnorm(n(), mean = mean_group1, sd = sd_group1),
rnorm(n(), mean = mean_group2, sd = sd_group2))) %>%
group_by(condition) %>%
mutate(value = b0 + b1 * condition + intercept + rnorm(n(), sd = sd_residual)) %>%
ungroup %>%
mutate(condition = as.factor(condition),
participant = as.factor(participant))Let’s fit the model:
# fit model
fit.variance = lmer(formula = value ~ 1 + group + condition + (1 | participant),
data = df.variance)
summary(fit.variance)Linear mixed model fit by REML. t-tests use Satterthwaite's method [
lmerModLmerTest]
Formula: value ~ 1 + group + condition + (1 | participant)
Data: df.variance
REML criterion at convergence: 232.7
Scaled residuals:
Min 1Q Median 3Q Max
-2.96291 -0.19619 0.03751 0.28317 1.45552
Random effects:
Groups Name Variance Std.Dev.
participant (Intercept) 17.12 4.137
Residual 13.74 3.706
Number of obs: 40, groups: participant, 20
Fixed effects:
Estimate Std. Error df t value Pr(>|t|)
(Intercept) -24.0018 3.3669 19.1245 -7.129 8.56e-07 ***
group 27.0696 2.2353 18.0000 12.110 4.36e-10 ***
condition1 0.5716 1.1720 19.0000 0.488 0.631
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr) group
group -0.929
condition1 -0.174 0.000Look at the data and model predictions:
fit.variance %>%
augment() %>%
clean_names() %>%
ggplot(data = .,
mapping = aes(x = condition,
y = value,
group = participant)) +
geom_point(alpha = 0.5) +
geom_line(alpha = 0.5) +
geom_point(aes(y = fitted),
color = "red") +
geom_line(aes(y = fitted),
color = "red")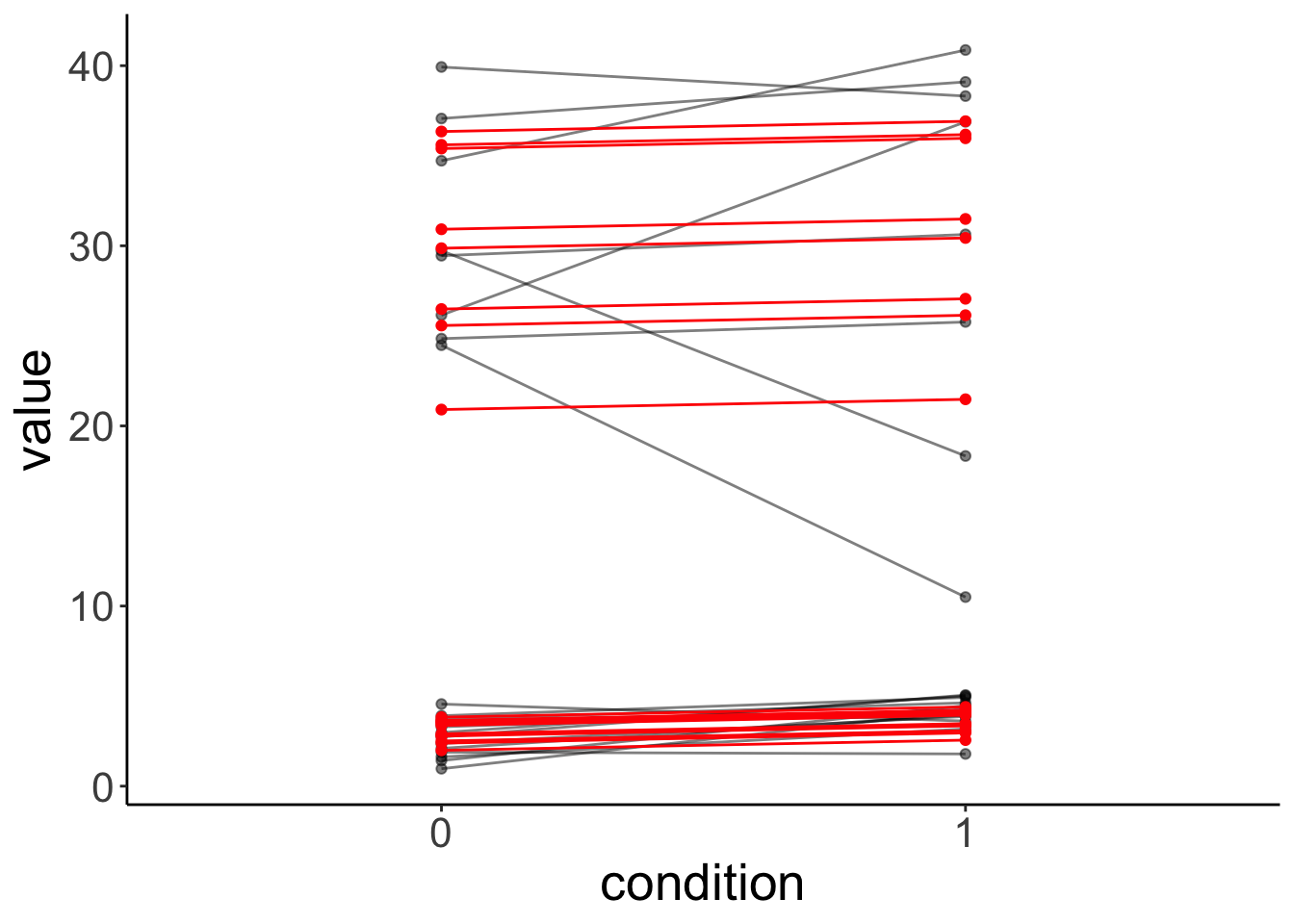
And the simulated data:
# simulated data
fit.variance %>%
simulate() %>%
bind_cols(df.mixed) %>%
ggplot(data = .,
mapping = aes(x = condition,
y = sim_1,
group = participant)) +
geom_line(alpha = 0.5) +
geom_point(alpha = 0.5)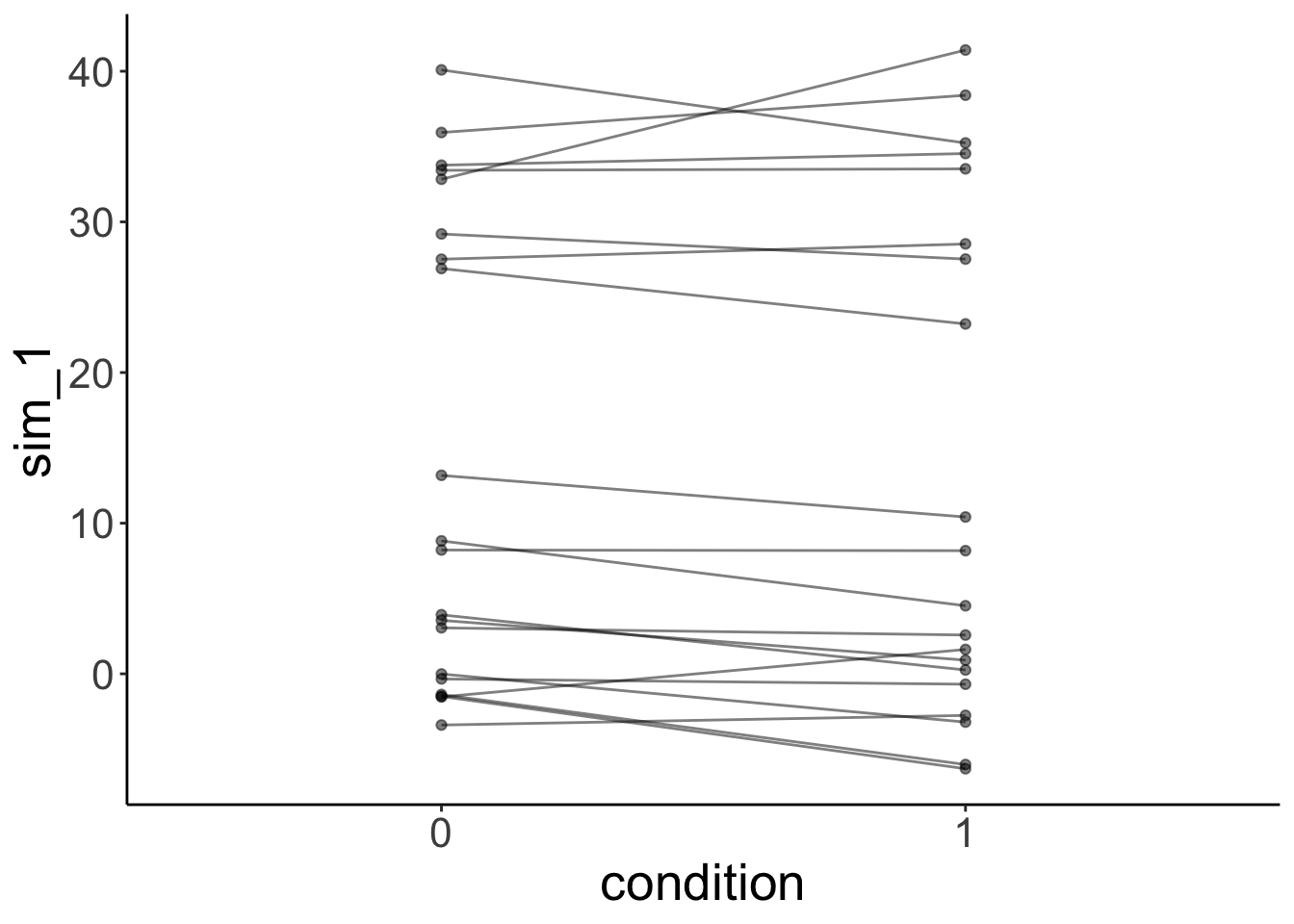
The lmer() fails here. It uses one normal distribution to model the variance between participants. It cannot account for the fact that the answers of one group of participants vary more than the answers from another groups of participants. Again, the simulated data doesn’t look like the original data, even though we did take the grouping into account.
We will later see that it’s straightforward in Bayesian models to explicitly model heterogeneity in variance.
20.6 Simulating different random effects structures
The examples below are taken from this post.
20.6.1 Two-level model
Figure 20.1: Two-level model
20.6.1.1 Conditional model
20.6.1.1.1 Cimulate the data
set.seed(1)
n_participants = 100
n_timepoints = 3
n_conditions = 2
p_condition = 0.5
b0 = 10
b1 = 10
sd_participant = 2
sd_residual = 1
df.data = tibble(participant = rep(1:n_participants, each = n_timepoints),
timepoint = rep(1:n_timepoints, times = n_participants),
intercept_participant = rep(rnorm(n_participants, sd = sd_participant),
each = n_timepoints)) %>%
group_by(participant) %>%
mutate(condition = rbinom(n = 1, size = 1, prob = p_condition)) %>%
ungroup() %>%
mutate(value = b0 + b1 * condition + intercept_participant +
rnorm(n_participants * n_timepoints, sd = sd_residual))20.6.1.1.2 Plot the data
df.plot = df.data %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint))
ggplot(data = df.plot,
mapping = aes(x = timepoint,
y = value,
group = participant)) +
geom_point(alpha = 0.5) +
geom_line(alpha = 0.5) +
facet_grid(~ condition) +
labs(x = "timepoint")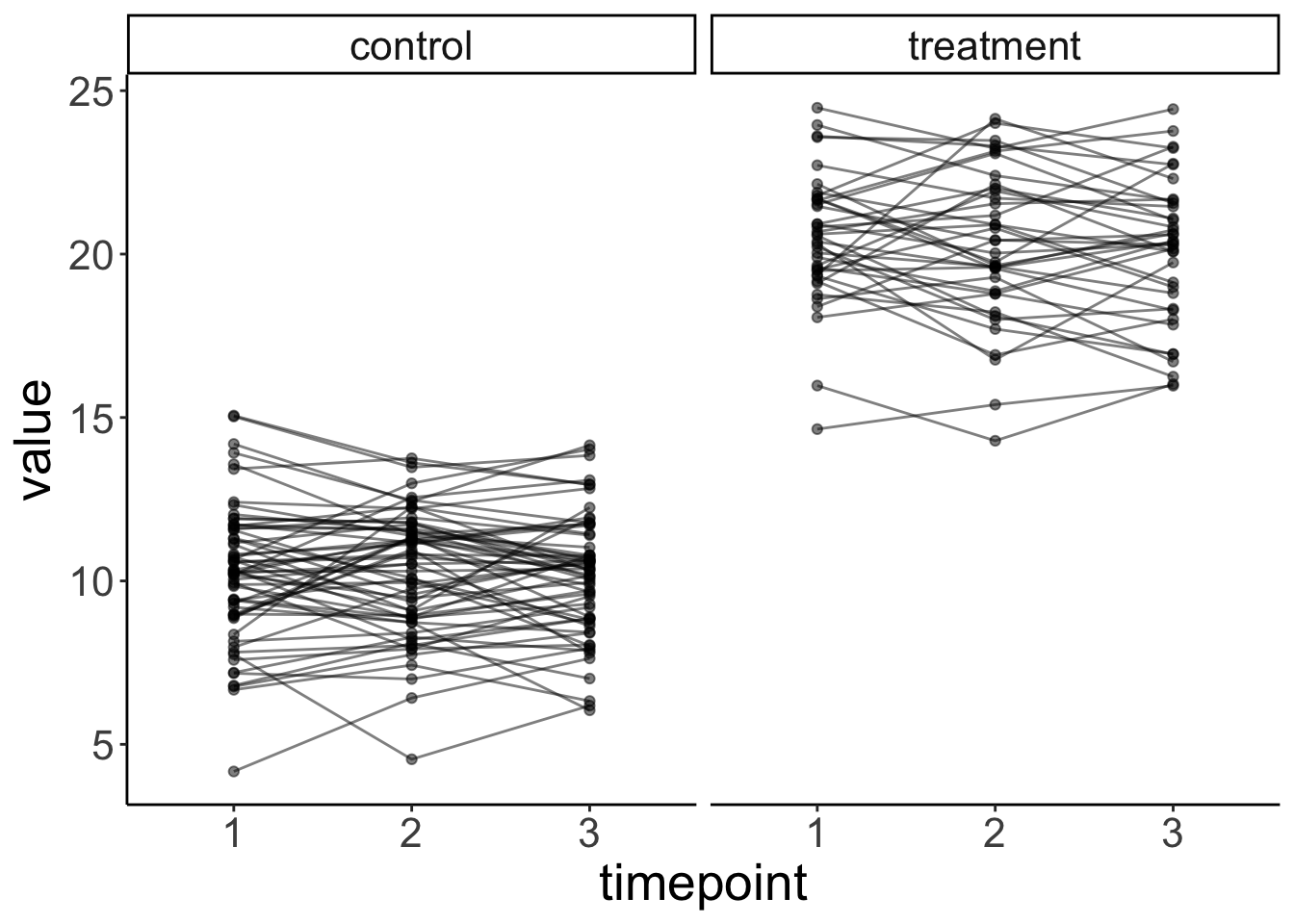
20.6.1.1.3 Fit the model
Linear mixed model fit by REML. t-tests use Satterthwaite's method [
lmerModLmerTest]
Formula: value ~ 1 + condition + (1 | participant)
Data: df.data
REML criterion at convergence: 1102
Scaled residuals:
Min 1Q Median 3Q Max
-2.30522 -0.57146 0.03152 0.56826 2.28135
Random effects:
Groups Name Variance Std.Dev.
participant (Intercept) 3.106 1.762
Residual 1.087 1.043
Number of obs: 300, groups: participant, 100
Fixed effects:
Estimate Std. Error df t value Pr(>|t|)
(Intercept) 10.2199 0.2365 98.0000 43.21 <2e-16 ***
condition 10.0461 0.3837 98.0000 26.18 <2e-16 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr)
condition -0.61620.6.1.1.4 Simulate and plot new data
set.seed(1)
fit %>%
simulate() %>%
bind_cols(df.data) %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint)) %>%
ggplot(data = .,
mapping = aes(x = timepoint,
y = sim_1,
group = participant)) +
geom_point(alpha = 0.5,
color = "blue") +
geom_line(alpha = 0.5,
color = "blue") +
facet_grid(~ condition) +
labs(x = "timepoint")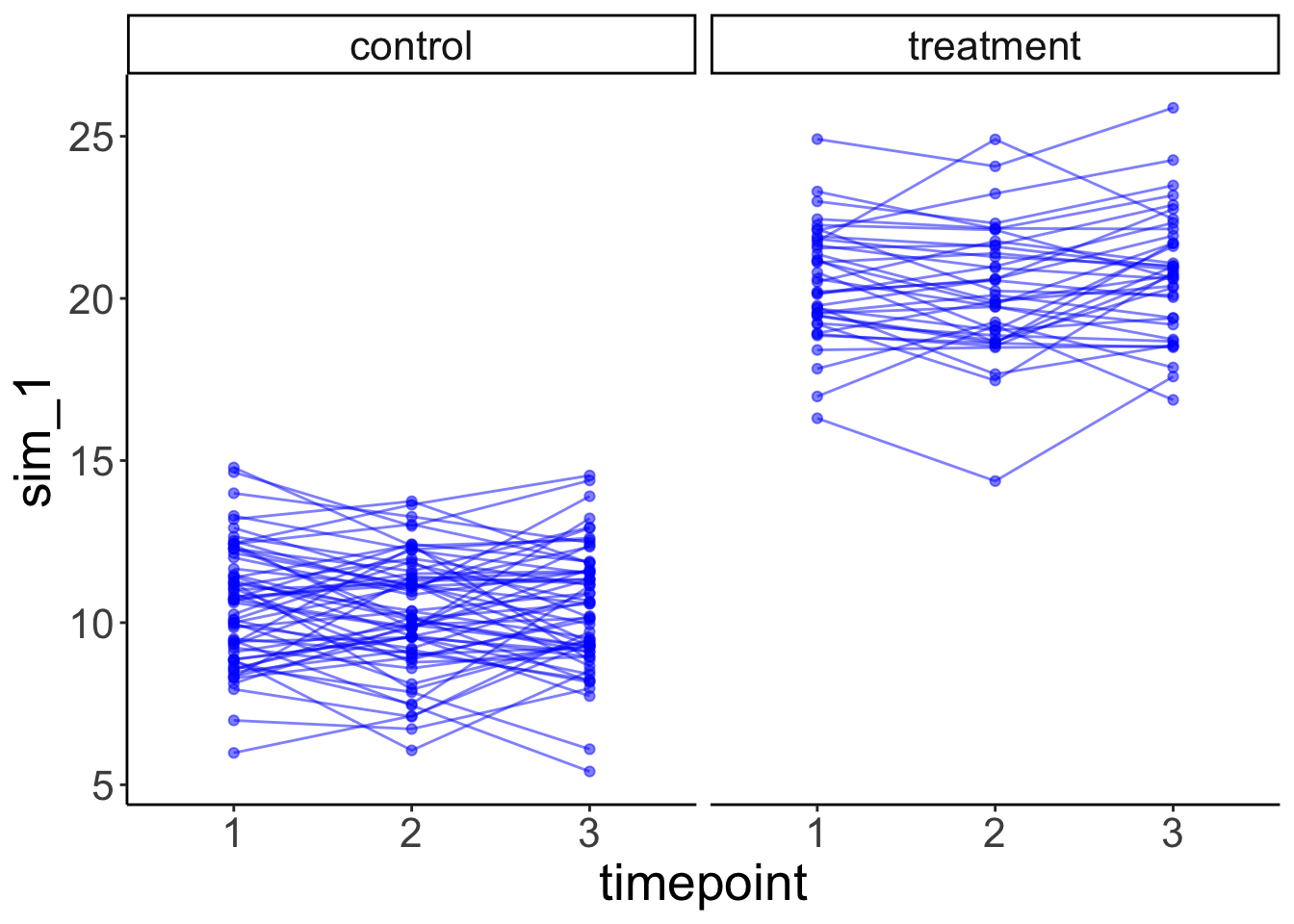
20.6.1.2 Conditional growth model
20.6.1.2.1 Simulate the data
set.seed(1)
n_participants = 100
n_timepoints = 3
n_conditions = 2
p_condition = 0.5
b0 = 10 # intercept
b1 = 10 # condition
b2 = 2 # time
b3 = 3 # interaction
sd_participant = 2
sd_time = 2
sd_residual = 1
df.data = tibble(participant = rep(1:n_participants, each = n_timepoints),
timepoint = rep(1:n_timepoints, times = n_participants),
intercept_participant = rep(rnorm(n_participants, sd = sd_participant),
each = n_timepoints),
time_participant = rep(rnorm(n_participants, sd = sd_time),
each = n_timepoints)) %>%
group_by(participant) %>%
mutate(condition = rbinom(n = 1, size = 1, prob = p_condition)) %>%
ungroup() %>%
mutate(value = b0 + intercept_participant +
b1 * condition +
(b2 + time_participant) * timepoint +
b3 * condition * timepoint +
rnorm(n_participants * n_timepoints, sd = sd_residual))20.6.1.2.2 Plot the data
df.plot = df.data %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint))
ggplot(data = df.plot,
mapping = aes(x = timepoint,
y = value,
group = participant)) +
geom_point(alpha = 0.5) +
geom_line(alpha = 0.5) +
facet_grid(~ condition) +
labs(x = "timepoint")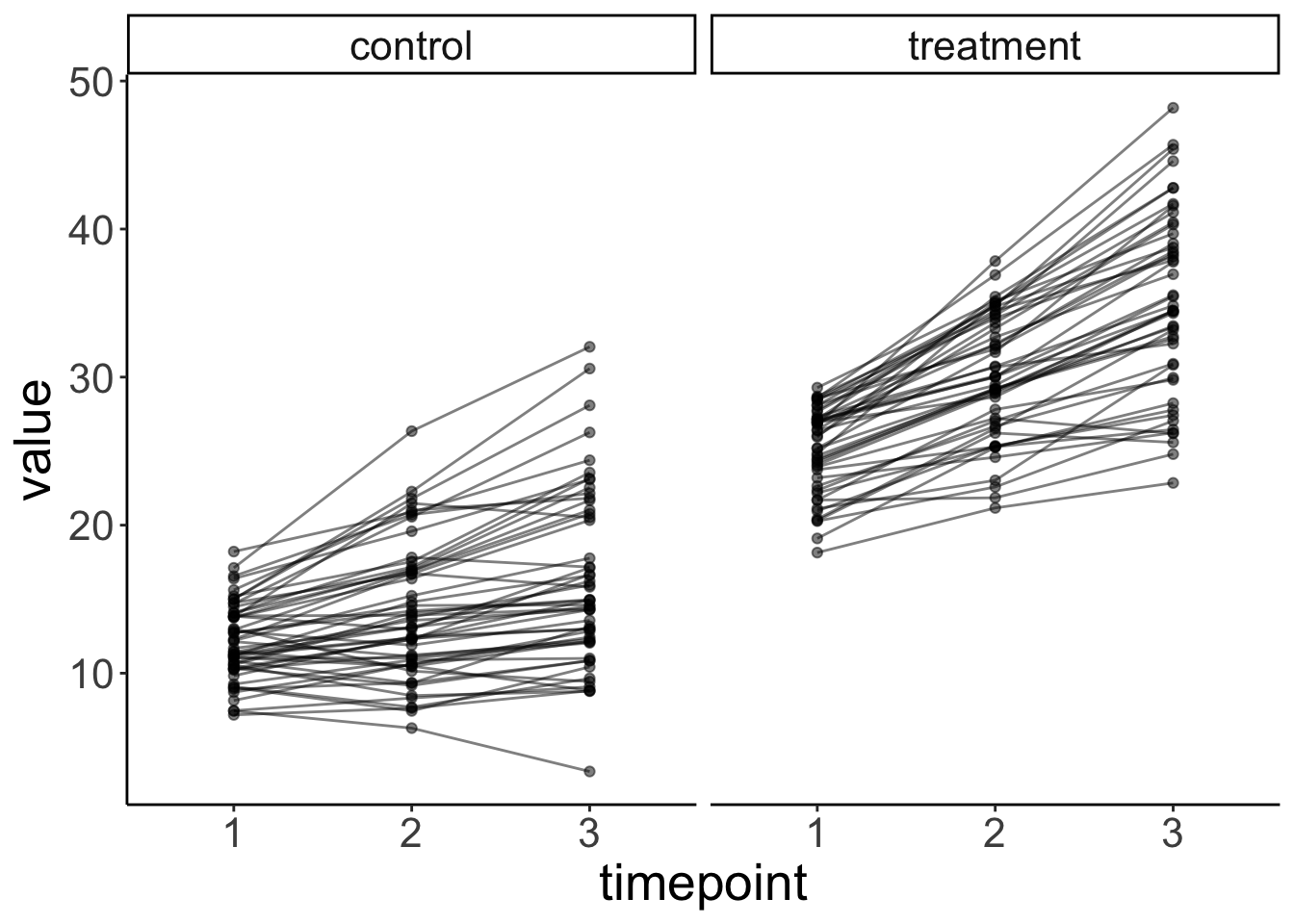
20.6.1.2.3 Fit the model
fit = lmer(formula = value ~ 1 + condition * timepoint + (1 + timepoint | participant),
data = df.data)
fit %>%
summary()Linear mixed model fit by REML. t-tests use Satterthwaite's method [
lmerModLmerTest]
Formula: value ~ 1 + condition * timepoint + (1 + timepoint | participant)
Data: df.data
REML criterion at convergence: 1360.3
Scaled residuals:
Min 1Q Median 3Q Max
-2.14633 -0.46360 0.03902 0.42302 2.82945
Random effects:
Groups Name Variance Std.Dev. Corr
participant (Intercept) 3.190 1.786
timepoint 3.831 1.957 -0.06
Residual 1.149 1.072
Number of obs: 300, groups: participant, 100
Fixed effects:
Estimate Std. Error df t value Pr(>|t|)
(Intercept) 10.0101 0.3328 98.0000 30.079 < 2e-16 ***
condition 10.0684 0.4854 98.0000 20.741 < 2e-16 ***
timepoint 2.0595 0.2883 97.9999 7.143 1.62e-10 ***
condition:timepoint 2.9090 0.4205 97.9999 6.917 4.76e-10 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr) condtn timpnt
condition -0.686
timepoint -0.266 0.182
cndtn:tmpnt 0.182 -0.266 -0.68620.6.1.2.4 Data with individual model predictions
df.plot = fit %>%
augment() %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint))
ggplot(data = df.plot,
mapping = aes(x = timepoint,
y = value,
group = participant)) +
# geom_point(alpha = 0.5) +
# geom_line(alpha = 0.5) +
geom_point(mapping = aes(y = .fitted),
alpha = 0.3,
color = "red") +
geom_line(mapping = aes(y = .fitted),
alpha = 0.3,
color = "red") +
facet_grid(~ condition) +
labs(x = "timepoint")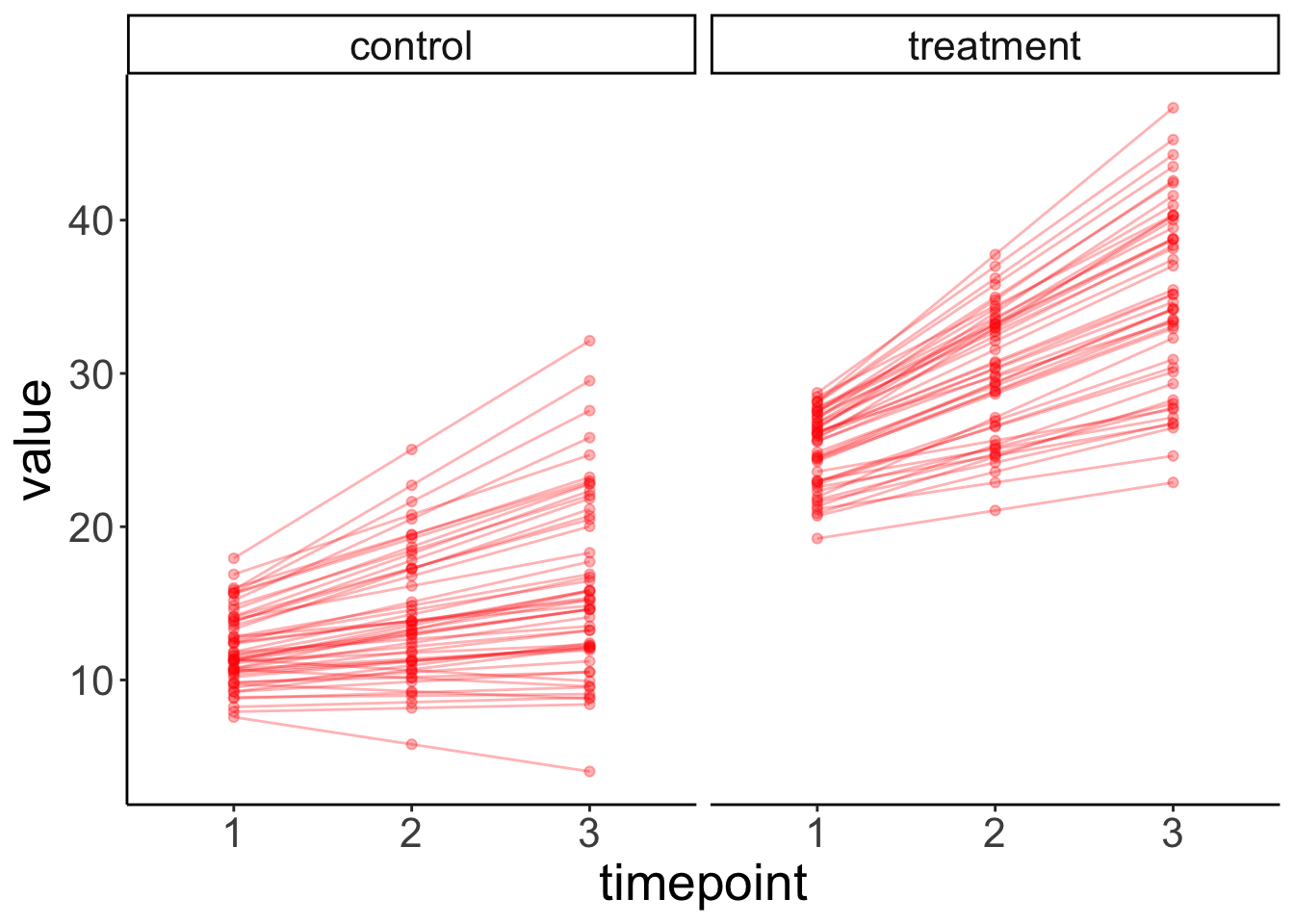
20.6.1.2.5 Data with overall model predictions
df.model = ggpredict(model = fit,
terms = c("timepoint", "condition"),
type = "fixed") %>%
rename(timepoint = x,
condition = group) %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint))
ggplot(data = df.plot,
mapping = aes(x = timepoint,
y = value,
group = participant)) +
geom_point(alpha = 0.2) +
geom_line(alpha = 0.2) +
geom_ribbon(data = df.model,
mapping = aes(ymin = conf.low,
ymax = conf.high,
y = predicted,
group = NA),
fill = "red",
alpha = 0.4) +
geom_point(data = df.model,
mapping = aes(y = predicted,
group = NA),
color = "red",
size = 3) +
geom_line(data = df.model,
mapping = aes(y = predicted,
group = NA),
color = "red",
linewidth = 1) +
facet_grid(~ condition) +
labs(x = "timepoint")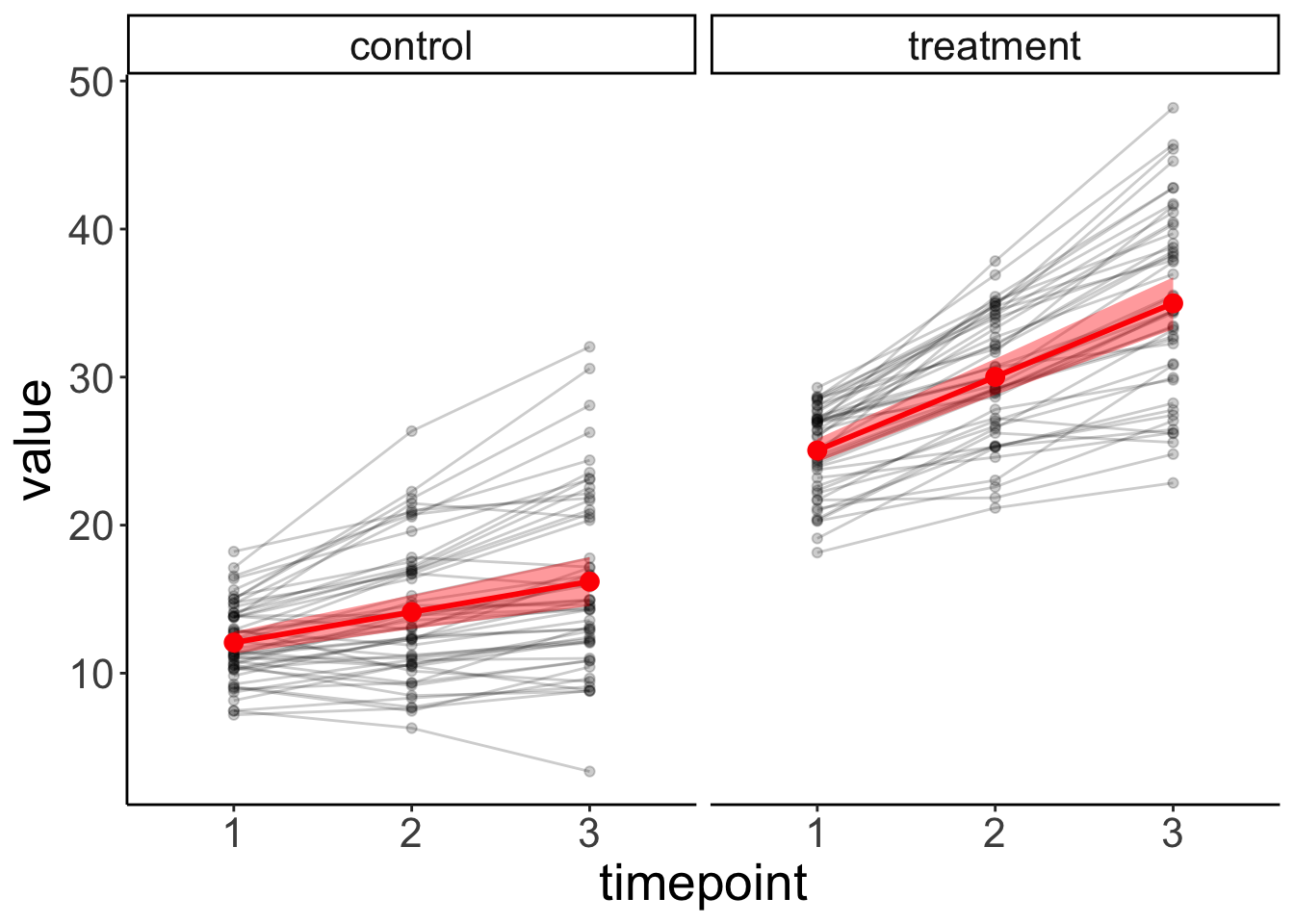
20.6.1.2.6 Simulate and plot new data
set.seed(1)
fit %>%
simulate() %>%
bind_cols(df.data) %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint)) %>%
ggplot(data = .,
mapping = aes(x = timepoint,
y = sim_1,
group = participant)) +
geom_point(alpha = 0.5,
color = "blue") +
geom_line(alpha = 0.5,
color = "blue") +
facet_grid(~ condition) +
labs(x = "timepoint")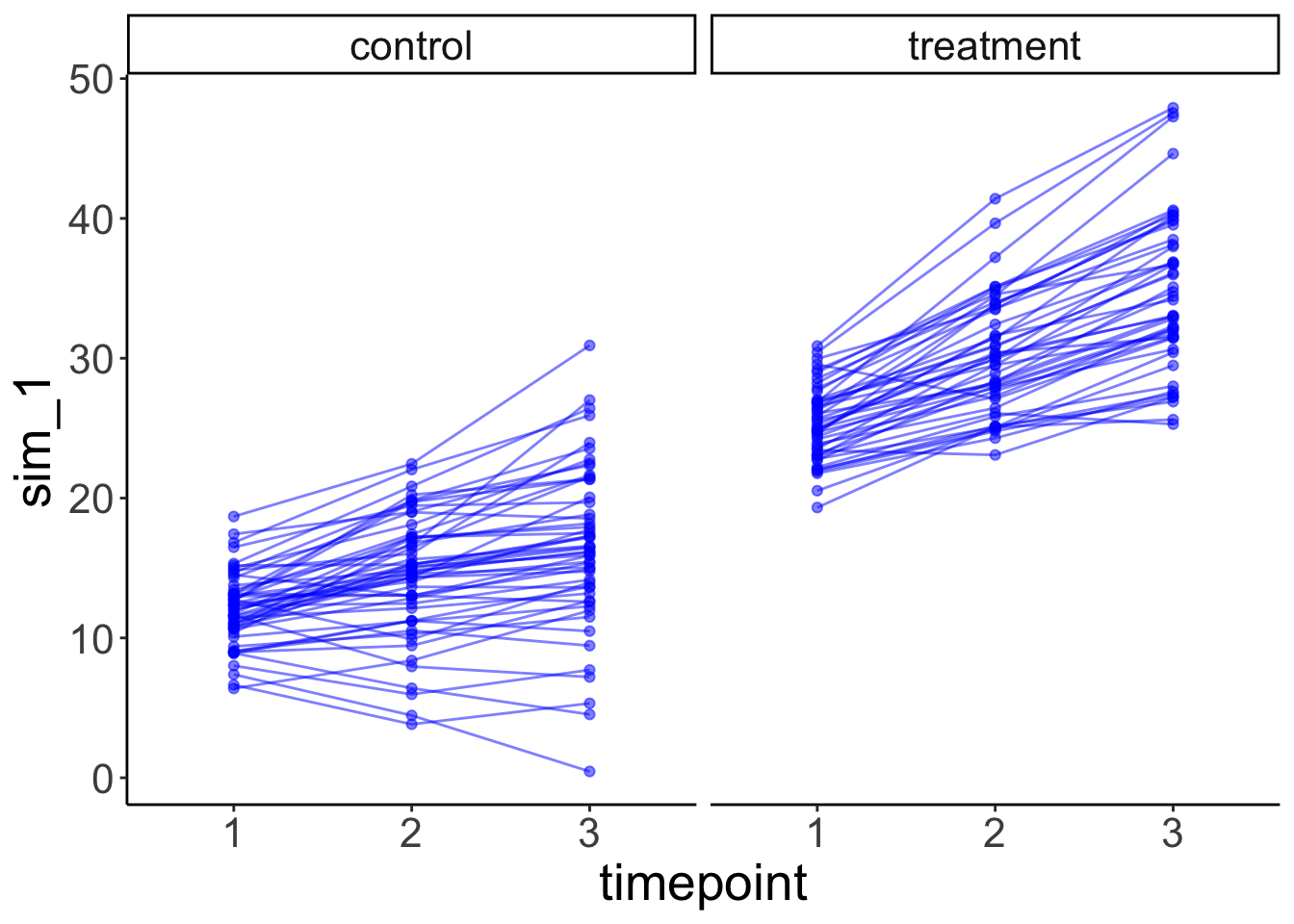
20.6.2 Three-level model
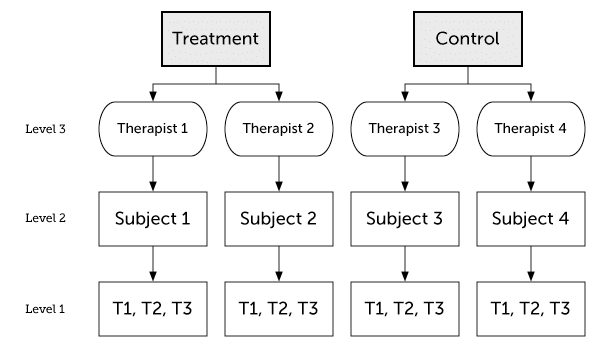
Figure 20.2: Three-level model
20.6.2.0.1 Simulate the data
set.seed(1)
n_participants = 100
n_therapists = 6
n_timepoints = 3
n_conditions = 2
p_condition = 0.5
b0 = 10 # intercept
b1 = 10 # condition
b2 = 2 # time
b3 = 3 # interaction
sd_intercept_therapist = 3
sd_intercept_participant = 2
sd_time_therapist = 2
sd_time_participant = 1
sd_residual = 1
df.data = tibble(participant = rep(1:n_participants, each = n_timepoints),
timepoint = rep(1:n_timepoints, times = n_participants),
intercept_participant = rep(rnorm(n_participants,
sd = sd_intercept_participant),
each = n_timepoints),
time_participant = rep(rnorm(n_participants, sd = sd_time_participant),
each = n_timepoints)) %>%
group_by(participant) %>%
mutate(condition = rbinom(n = 1, size = 1, prob = p_condition),
therapist = ifelse(condition == 0, sample(x = 1:(n_therapists/2),
size = 1),
sample(x = ((n_therapists/2)+1):n_therapists,
size = 1))) %>%
ungroup() %>%
group_by(therapist) %>%
mutate(intercept_therapist = rnorm(1, sd = sd_intercept_therapist),
time_therapist = rnorm(1, sd = sd_time_therapist)) %>%
ungroup() %>%
mutate(value = b0 + intercept_therapist + intercept_participant +
b1 * condition +
(b2 + time_therapist + time_participant) * timepoint +
b3 * condition * timepoint +
rnorm(n_participants * n_timepoints, sd = sd_residual))20.6.2.0.2 Plot the data
df.plot = df.data %>%
mutate(condition = factor(condition,
levels = c(0, 1),
labels = c("control", "treatment")),
timepoint = as.factor(timepoint),
therapist = as.factor(therapist))
ggplot(data = df.plot,
mapping = aes(x = timepoint,
y = value,
group = participant,
color = therapist)) +
geom_point(alpha = 0.5) +
geom_line(alpha = 0.5) +
facet_grid(~ condition) +
labs(x = "timepoint")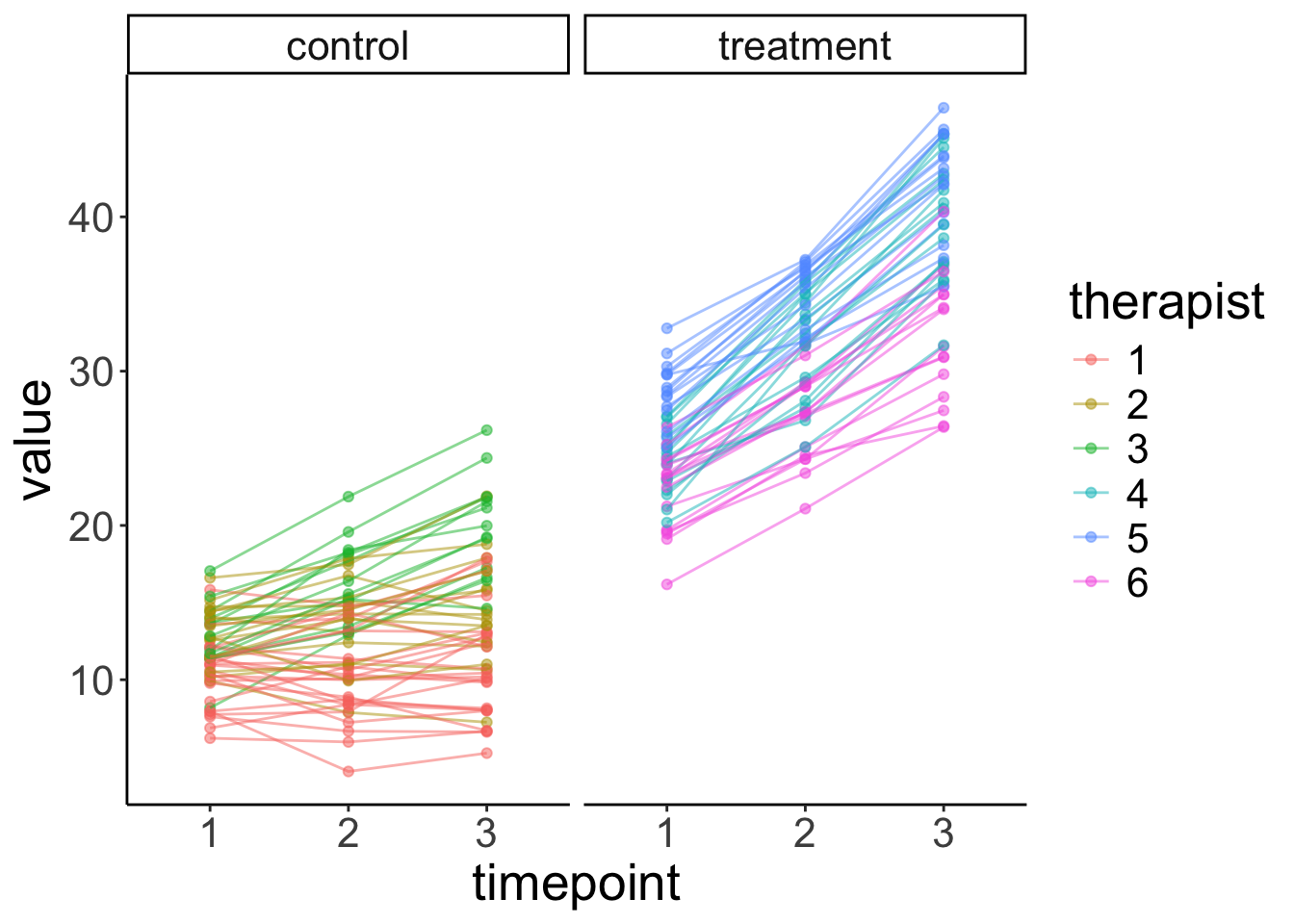
20.6.2.0.3 Fit the model
fit = lmer(formula = value ~ 1 + condition * timepoint +
(1 + timepoint | therapist) +
(1 + timepoint | therapist:participant),
data = df.data)
fit %>%
summary()Linear mixed model fit by REML. t-tests use Satterthwaite's method [
lmerModLmerTest]
Formula: value ~ 1 + condition * timepoint + (1 + timepoint | therapist) +
(1 + timepoint | therapist:participant)
Data: df.data
REML criterion at convergence: 1237.9
Scaled residuals:
Min 1Q Median 3Q Max
-2.02926 -0.51103 0.01576 0.48074 2.12179
Random effects:
Groups Name Variance Std.Dev. Corr
therapist:participant (Intercept) 2.1361 1.4616
timepoint 0.8205 0.9058 0.33
therapist (Intercept) 5.6350 2.3738
timepoint 2.4175 1.5548 -0.21
Residual 1.0515 1.0254
Number of obs: 300, groups: therapist:participant, 100; therapist, 6
Fixed effects:
Estimate Std. Error df t value Pr(>|t|)
(Intercept) 10.5078 1.4037 3.9706 7.486 0.00175 **
condition 7.7672 1.9866 3.9825 3.910 0.01754 *
timepoint 1.5160 0.9126 3.9765 1.661 0.17244
condition:timepoint 5.0489 1.2913 3.9845 3.910 0.01752 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr) condtn timpnt
condition -0.707
timepoint -0.208 0.147
cndtn:tmpnt 0.147 -0.208 -0.70720.7 Bootstrapping
Bootstrapping is a good way to estimate our uncertainty on the parameter estimates in the model.
20.7.1 Linear model
Let’s briefly review how to do bootstrapping in a simple linear model.
(Intercept) days
252.32070 10.32766 # bootstrapping
df.boot = df.sleep %>%
bootstrap(n = 100,
id = "id") %>%
mutate(fit = map(.x = strap,
.f = ~ lm(formula = reaction ~ 1 + days,
data = .x)),
tidy = map(.x = fit,
.f = tidy)) %>%
unnest(tidy) %>%
select(id, term, estimate) %>%
spread(term, estimate) %>%
clean_names() Let’s illustrate the linear model with a confidence interval (making parametric assumptions using the t-distribution).
ggplot(data = df.sleep,
mapping = aes(x = days,
y = reaction)) +
geom_smooth(method = "lm") +
geom_point(alpha = 0.3)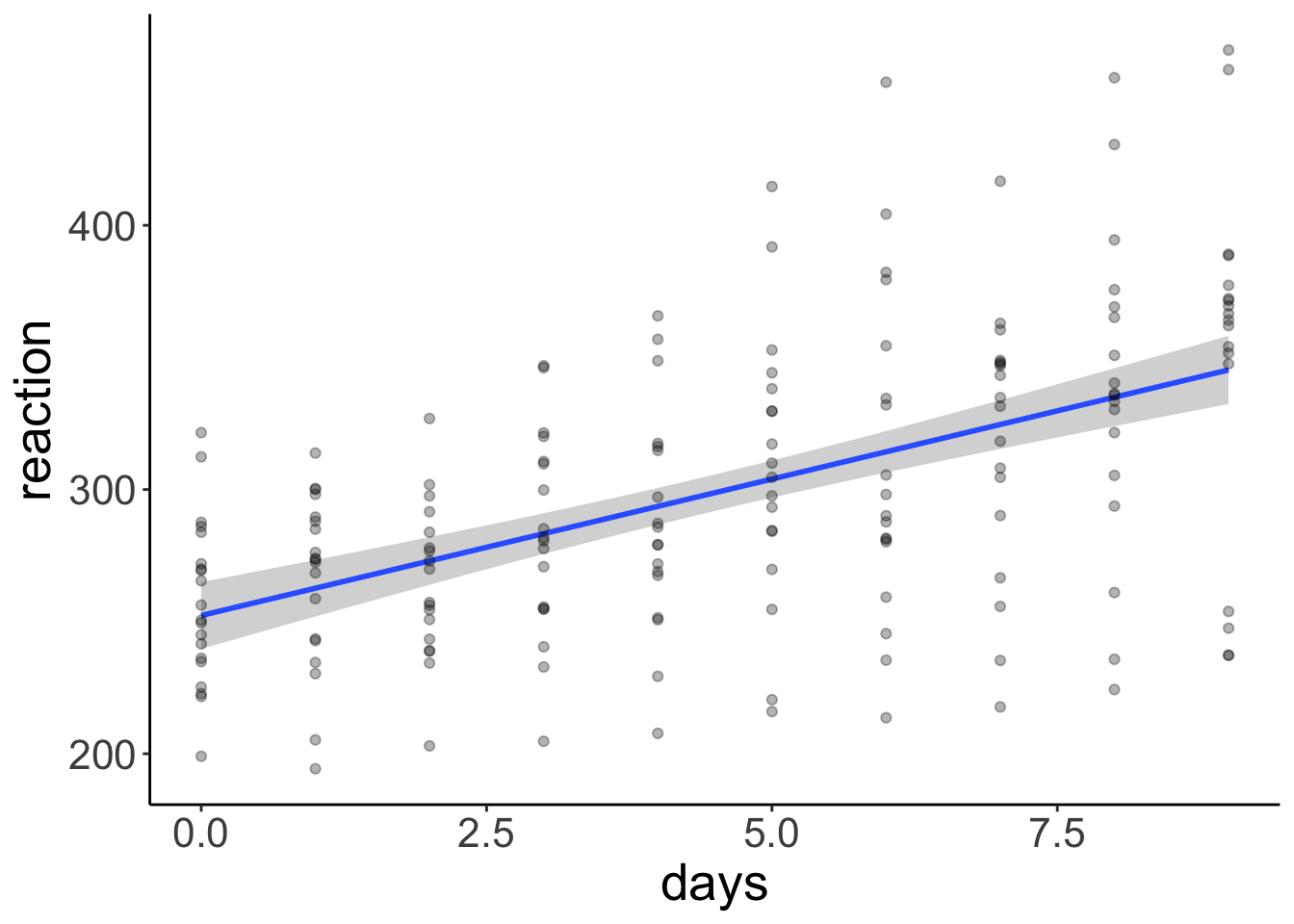
And let’s compare this with the different regression lines that we get out of our bootstrapped samples:
ggplot(data = df.sleep,
mapping = aes(x = days,
y = reaction)) +
geom_abline(data = df.boot,
aes(intercept = intercept,
slope = days,
group = id),
alpha = 0.1) +
geom_point(alpha = 0.3)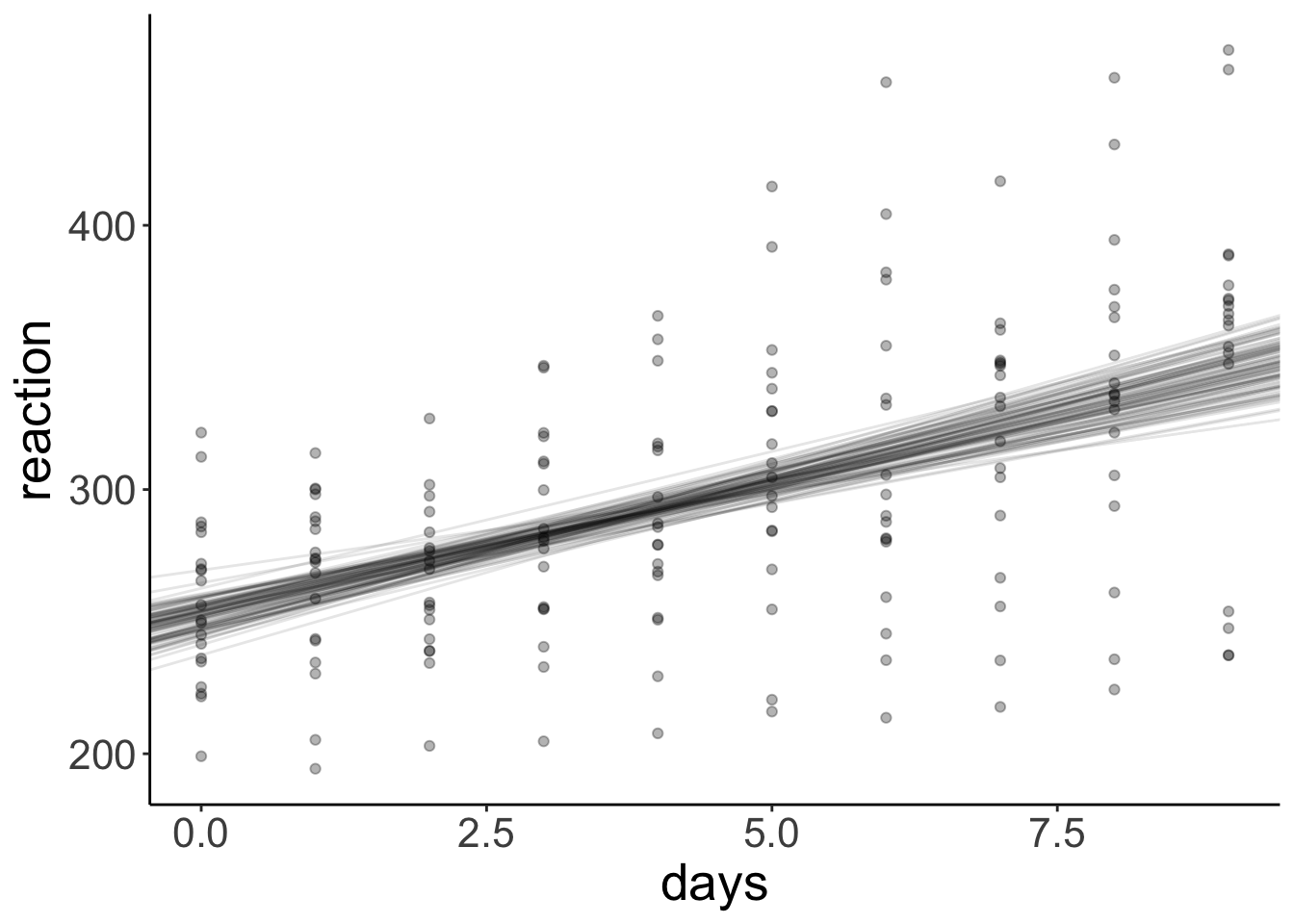
20.7.2 Linear mixed effects model
For the linear mixed effects model, we can use the bootmer() function to do bootstrapping.
set.seed(1)
# fit the model
fit.lmer = lmer(formula = reaction ~ 1 + days + (1 + days | subject),
data = df.sleep)
# bootstrap parameter estimates
boot.lmer = bootMer(fit.lmer,
FUN = fixef,
nsim = 100)
# compute confidence interval
boot.ci(boot.lmer,
index = 2,
type = "perc")BOOTSTRAP CONFIDENCE INTERVAL CALCULATIONS
Based on 100 bootstrap replicates
CALL :
boot.ci(boot.out = boot.lmer, type = "perc", index = 2)
Intervals :
Level Percentile
95% ( 7.26, 13.79 )
Calculations and Intervals on Original Scale
Some percentile intervals may be unstableLet’s plot the distribution of estimates.
# plot distribution of estimates
boot.lmer$t %>%
as_tibble() %>%
clean_names() %>%
mutate(id = 1:n()) %>%
pivot_longer(cols = -id,
names_to = "index",
values_to = "value") %>%
ggplot(data = .,
mapping = aes(x = value)) +
geom_density() +
facet_grid(cols = vars(index),
scales = "free") +
coord_cartesian(expand = F)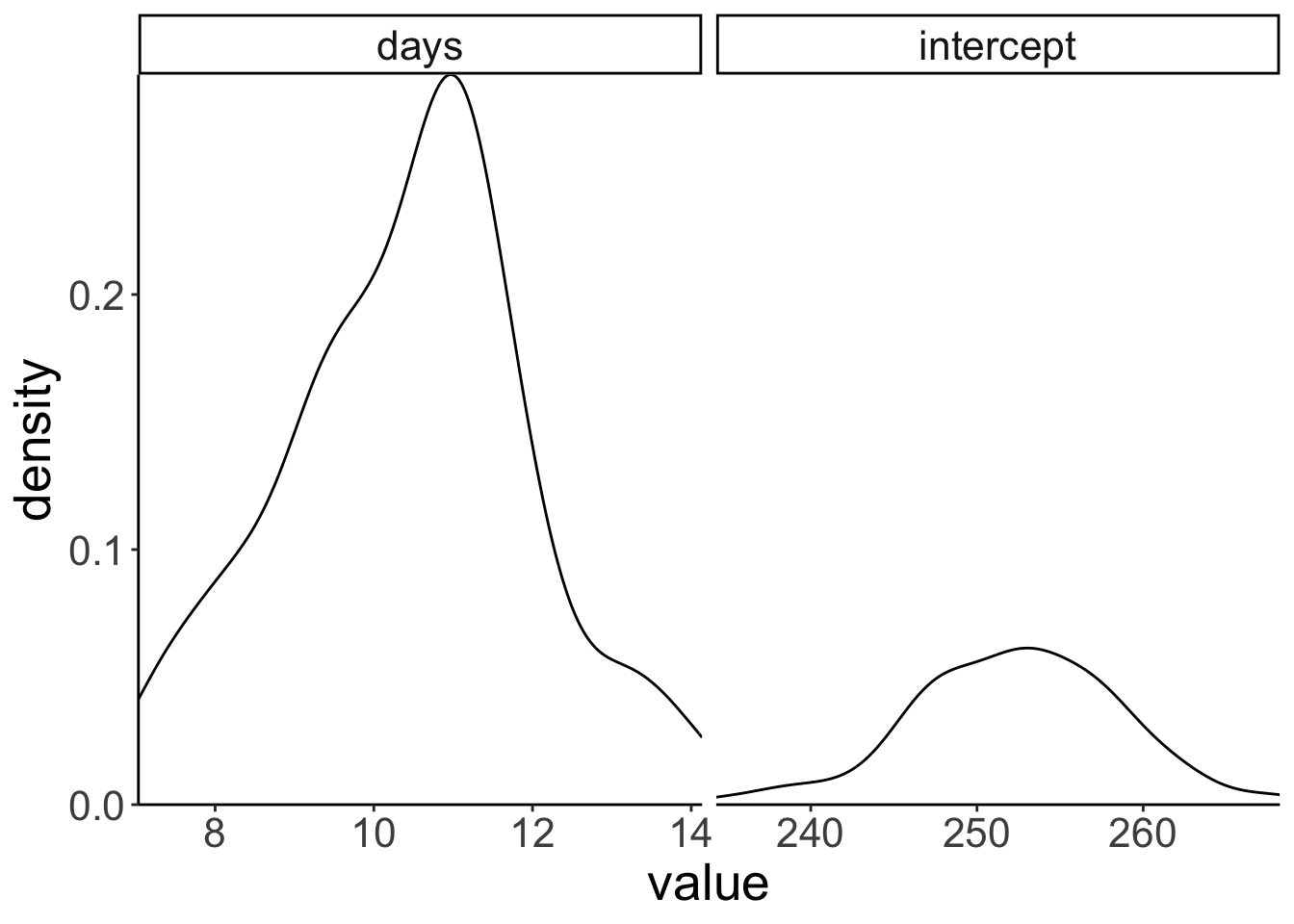
And let’s look at the predictions together with the data.
df.boot_lmer = boot.lmer$t %>%
as_tibble() %>%
clean_names() %>%
mutate(id = 1:n())
ggplot(data = df.sleep,
mapping = aes(x = days,
y = reaction)) +
geom_abline(data = df.boot_lmer,
aes(intercept = intercept,
slope = days,
group = id),
alpha = 0.1) +
geom_point(alpha = 0.3)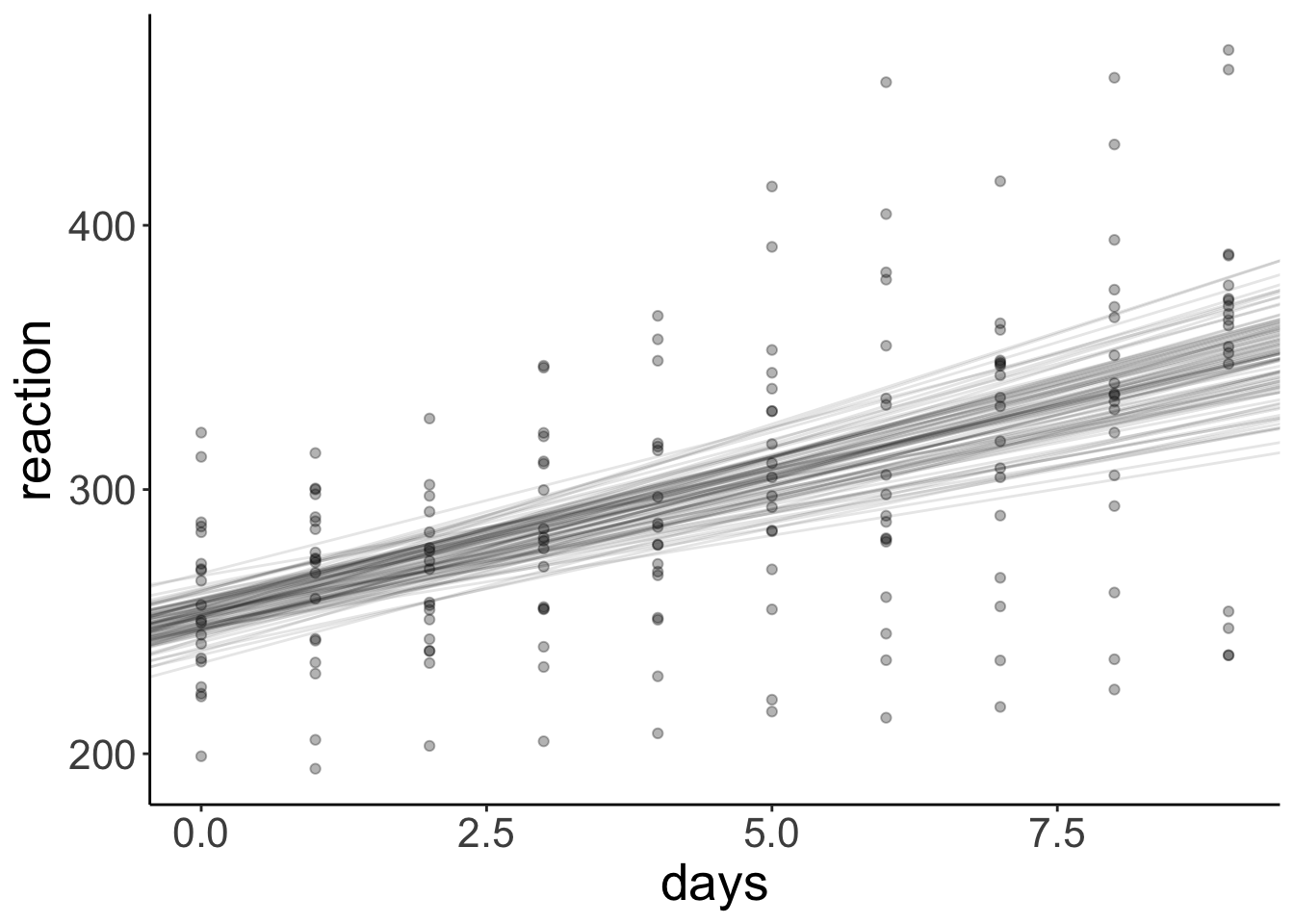
As you’ll notice, once we take the dependence in the data into account, the bootstrapped confidence interval is wider than when we ignore the dependence.
20.8 Logistic mixed effects model
Just like we can build linear mixed effects models using lmer() instead of lm(), we can also build a logistic mixed effects regression using glmer() instead of glm().
Let’s read in some data:
# load bdf data set from nlme package
data(bdf, package = "nlme")
df.language = bdf %>%
clean_names() %>%
filter(repeatgr != 2) %>%
mutate(repeatgr = repeatgr %>%
as.character() %>%
as.numeric())
rm(bdf)Fit the model, and print out the results:
fit = glmer(repeatgr ~ 1 + ses + minority + (1 | school_nr),
data = df.language,
family = "binomial")
fit %>%
summary()Generalized linear mixed model fit by maximum likelihood (Laplace
Approximation) [glmerMod]
Family: binomial ( logit )
Formula: repeatgr ~ 1 + ses + minority + (1 | school_nr)
Data: df.language
AIC BIC logLik deviance df.resid
1659.1 1682.1 -825.6 1651.1 2279
Scaled residuals:
Min 1Q Median 3Q Max
-0.9235 -0.4045 -0.3150 -0.2249 5.8372
Random effects:
Groups Name Variance Std.Dev.
school_nr (Intercept) 0.2489 0.4989
Number of obs: 2283, groups: school_nr, 131
Fixed effects:
Estimate Std. Error z value Pr(>|z|)
(Intercept) -0.506280 0.197568 -2.563 0.01039 *
ses -0.060086 0.007524 -7.986 1.39e-15 ***
minorityY 0.673605 0.238655 2.823 0.00477 **
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr) ses
ses -0.898
minorityY -0.308 0.208To visualize the results, we can use the ggeffects package.
You are calculating adjusted predictions on the population-level (i.e.
`type = "fixed"`) for a *generalized* linear mixed model.
This may produce biased estimates due to Jensen's inequality. Consider
setting `bias_correction = TRUE` to correct for this bias.
See also the documentation of the `bias_correction` argument.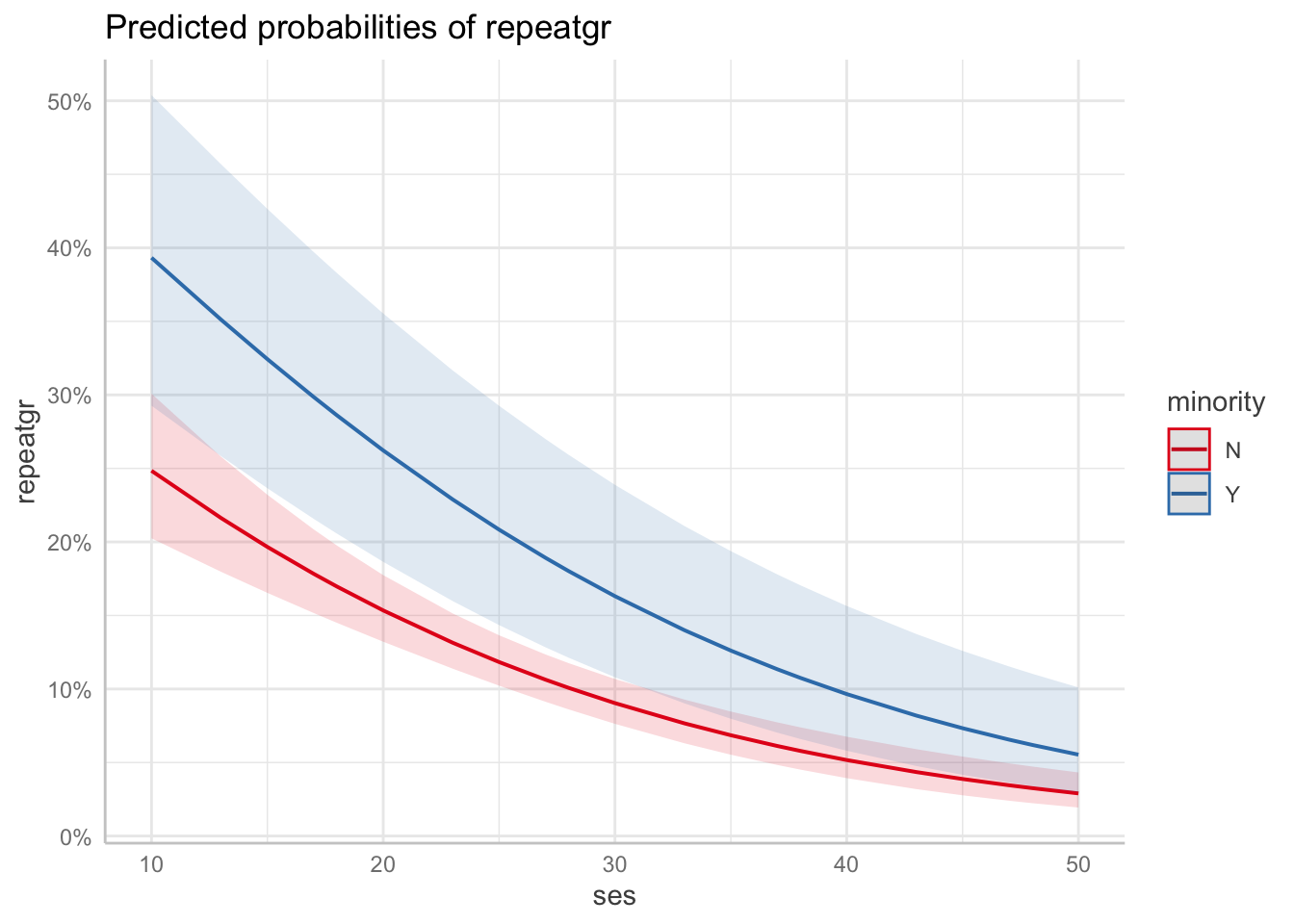
And for significance testing, we can use the the joint_tests() function from the “emmeans” package
glmer(formula = repeatgr ~ 1 + ses + minority + (1 | school_nr),
data = df.language,
family = "binomial") %>%
joint_tests() model term df1 df2 F.ratio Chisq p.value
ses 1 Inf 63.784 63.784 <.0001
minority 1 Inf 7.967 7.967 0.0048The results show that there was both a significant effect of ses and of minority.
Note: This post here says a little more about the relationship of the F.ratio in the joint_tests() function, and what a likelihood ratio test yields. In short, it’s roughly the same thing.
If you’d like to compute the likelihood ratio test, a convenient way of doing so is by using the mixed() function from the “afex” package.
mixed(formula = repeatgr ~ 1 + ses + minority + (1 | school_nr),
family = "binomial",
data = df.language,
method = "LRT")Contrasts set to contr.sum for the following variables: minority, school_nrNumerical variables NOT centered on 0: ses
If in interactions, interpretation of lower order (e.g., main) effects difficult.Mixed Model Anova Table (Type 3 tests, LRT-method)
Model: repeatgr ~ 1 + ses + minority + (1 | school_nr)
Data: df.language
Df full model: 4
Effect df Chisq p.value
1 ses 1 75.39 *** <.001
2 minority 1 7.53 ** .006
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '+' 0.1 ' ' 1And we can compare that the model comparison approach gives us the same result:
fit_a = glmer(repeatgr ~ 1 + ses + minority + (1 | school_nr),
data = df.language,
family = "binomial")
# dropping ses as a predictor
fit_c = glmer(repeatgr ~ 1 + minority + (1 | school_nr),
data = df.language,
family = "binomial")
anova(fit_a, fit_c, test = "LRT")Data: df.language
Models:
fit_c: repeatgr ~ 1 + minority + (1 | school_nr)
fit_a: repeatgr ~ 1 + ses + minority + (1 | school_nr)
npar AIC BIC logLik deviance Chisq Df Pr(>Chisq)
fit_c 3 1732.5 1749.7 -863.27 1726.5
fit_a 4 1659.1 1682.1 -825.57 1651.1 75.395 1 < 2.2e-16 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 120.9 Session info
Information about this R session including which version of R was used, and what packages were loaded.
R version 4.4.2 (2024-10-31)
Platform: aarch64-apple-darwin20
Running under: macOS Sequoia 15.2
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.4-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.12.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: America/Los_Angeles
tzcode source: internal
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] lubridate_1.9.3 forcats_1.0.0 stringr_1.5.1
[4] dplyr_1.1.4 purrr_1.0.2 readr_2.1.5
[7] tidyr_1.3.1 tibble_3.2.1 ggplot2_3.5.1
[10] tidyverse_2.0.0 emmeans_1.10.6 ggeffects_2.0.0
[13] boot_1.3-31 modelr_0.1.11 datarium_0.1.0
[16] car_3.1-3 carData_3.0-5 afex_1.4-1
[19] lme4_1.1-35.5 Matrix_1.7-1 broom.mixed_0.2.9.6
[22] janitor_2.2.1 kableExtra_1.4.0 knitr_1.49
loaded via a namespace (and not attached):
[1] gridExtra_2.3 rlang_1.1.4 magrittr_2.0.3
[4] snakecase_0.11.1 furrr_0.3.1 compiler_4.4.2
[7] mgcv_1.9-1 systemfonts_1.1.0 vctrs_0.6.5
[10] reshape2_1.4.4 pkgconfig_2.0.3 crayon_1.5.3
[13] fastmap_1.2.0 backports_1.5.0 labeling_0.4.3
[16] utf8_1.2.4 rmarkdown_2.29 tzdb_0.4.0
[19] haven_2.5.4 nloptr_2.1.1 bit_4.0.5
[22] xfun_0.49 cachem_1.1.0 jsonlite_1.8.8
[25] broom_1.0.7 parallel_4.4.2 cluster_2.1.6
[28] R6_2.5.1 RColorBrewer_1.1-3 bslib_0.7.0
[31] stringi_1.8.4 parallelly_1.37.1 rpart_4.1.23
[34] jquerylib_0.1.4 numDeriv_2016.8-1.1 estimability_1.5.1
[37] Rcpp_1.0.13 bookdown_0.42 base64enc_0.1-3
[40] splines_4.4.2 nnet_7.3-19 timechange_0.3.0
[43] tidyselect_1.2.1 rstudioapi_0.16.0 abind_1.4-5
[46] yaml_2.3.10 sjlabelled_1.2.0 codetools_0.2-20
[49] listenv_0.9.1 lattice_0.22-6 lmerTest_3.1-3
[52] plyr_1.8.9 withr_3.0.2 coda_0.19-4.1
[55] evaluate_0.24.0 foreign_0.8-87 future_1.33.2
[58] xml2_1.3.6 pillar_1.9.0 checkmate_2.3.1
[61] insight_1.0.0 generics_0.1.3 vroom_1.6.5
[64] hms_1.1.3 munsell_0.5.1 scales_1.3.0
[67] minqa_1.2.7 globals_0.16.3 xtable_1.8-4
[70] glue_1.8.0 Hmisc_5.2-1 tools_4.4.2
[73] data.table_1.15.4 mvtnorm_1.2-5 grid_4.4.2
[76] datawizard_0.13.0 colorspace_2.1-0 nlme_3.1-166
[79] htmlTable_2.4.2 Formula_1.2-5 cli_3.6.3
[82] fansi_1.0.6 viridisLite_0.4.2 svglite_2.1.3
[85] gtable_0.3.5 sass_0.4.9 digest_0.6.36
[88] pbkrtest_0.5.3 farver_2.1.2 htmlwidgets_1.6.4
[91] htmltools_0.5.8.1 lifecycle_1.0.4 bit64_4.0.5
[94] MASS_7.3-64